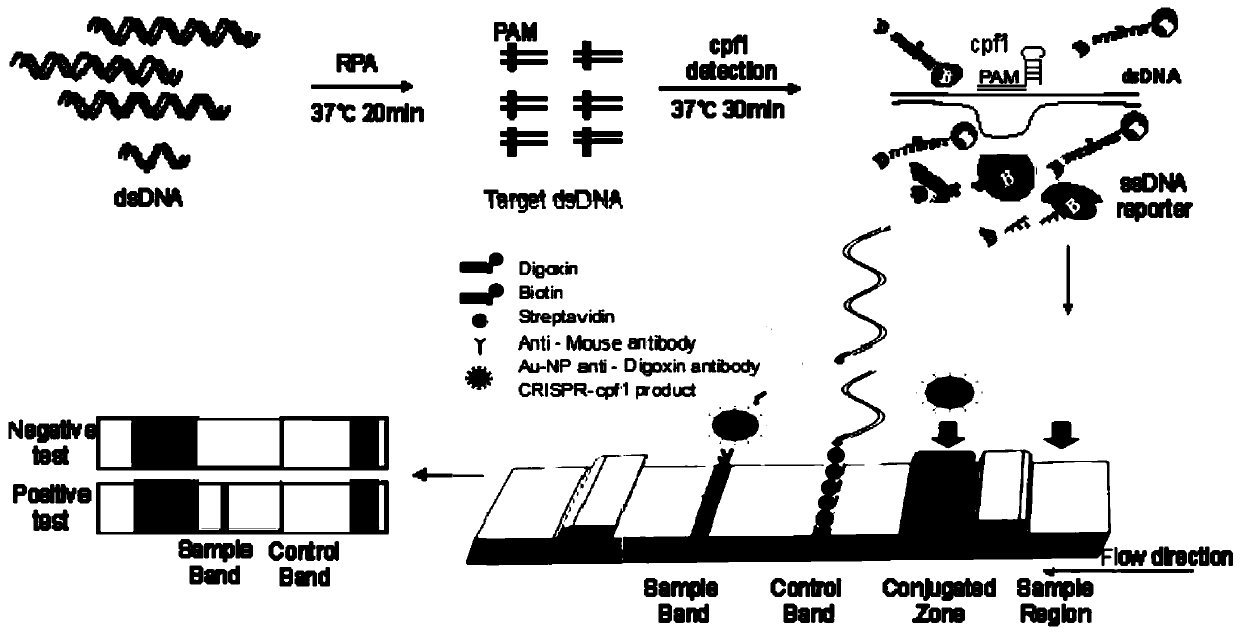
Cpf1 reagent kit and detection method for quickly detecting nucleic acid of African swine fever virus
An African swine fever virus and detection method technology, applied in the biological field, can solve problems such as expensive and unsuitable for on-site detection in pig farms, and achieve the effects of simple detection method, convenient and quick result interpretation, and high-sensitivity visual detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0048] Implementation Case 1: Rapid and Sensitive Detection of African Swine Fever Virus p72 Gene Fragment
[0049] 1.1 Nucleic acid preparation
[0050] In this case, the p72 gene fragment of African swine fever virus is based on the Genotype II subtype sequence, and the C-terminal 454bp SEQ NO.1 was synthesized by Nanjing GenScript Company and constructed into the pUC57 vector, named pUC57-ASFV-p72.
[0051] Using the RPA amplification primers RPA-F1SEQ NO.2 and RPA-R1SEQ NO.3 in the present invention, referring to the RPA isothermal amplification operation steps, amplified to obtain the sample to be detected. The specific operation is as follows:
[0052] In the case of sensitivity detection, the molecular weight of the pUC57-ASFV-P72 plasmid was calculated, and a 10-fold serial dilution was performed to obtain 2*e10, 2*e9, 2*e8, 2*e7, 2*e6, 2*e5 per microliter , 2*e4, 2*e3, 2*e2, 2*e1 and 2*e0 copy number (copy / μL). 1 μL of gradient dilution samples were subjected to RP...
Embodiment example 2
[0073] Implementation case 2: Rapid genotype identification of African swine fever virus P72 gene fragment
[0074] The rapid detection of African swine fever virus genotype is helpful to understand the source of virus infection and trace the route of virus transmission in detail, and is of great significance to the prevention and control of the virus. In this implementation case, ASFV subtype I, II and III are taken as examples to demonstrate the application of the Cpf1 detection kit of the present invention to type African swine fever virus.
[0075] Based on the fact that Cpf1 performs highly specific recognition and cutting of the target sequence under the guidance of crRNA, a hypothesis is proposed: there are differences in nucleic acid sequences of different genotypes of ASFV, which can lead to the inability of the Cpf1 cutting system to efficiently recognize, manifested as low detection fluorescence values or colloidal gold The test strip has no detection strip. Base...
Embodiment example 3
[0082] Implementation Case 3: Rapid ASFV Detection of Nucleic Acids in Clinical Tissue Samples
[0083] In this implementation case, ASFV rapid detection of nucleic acid in clinical tissue samples was performed. All samples and operations were completed in the national African swine fever regional laboratory, and all operations were carried out in strict accordance with relevant laws and regulations and relevant regulations of the Ministry of Agriculture.
[0084] The clinical DNA samples in this case came from the national African swine fever regional laboratory, including pig tissue samples collected from pig farms infected with African swine fever virus, including 6 tissue organs of lung, spleen, kidney, intestine, heart and liver 12 samples. The total DNA of the tissue was extracted, and qPCR detection and Cpf1 detection were performed respectively. In this example, a rapid nucleic acid release agent purchased from Novizyme was used to obtain pretreated nucleic acid. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com