BAS-ELISA kit for detecting hog cholera virus Erns and E2 antigen
A swine fever virus and kit technology, applied in the field of biotechnology and animal infectious disease diagnosis research, can solve problems such as false negative results, achieve stable results, convenient use, and improve sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
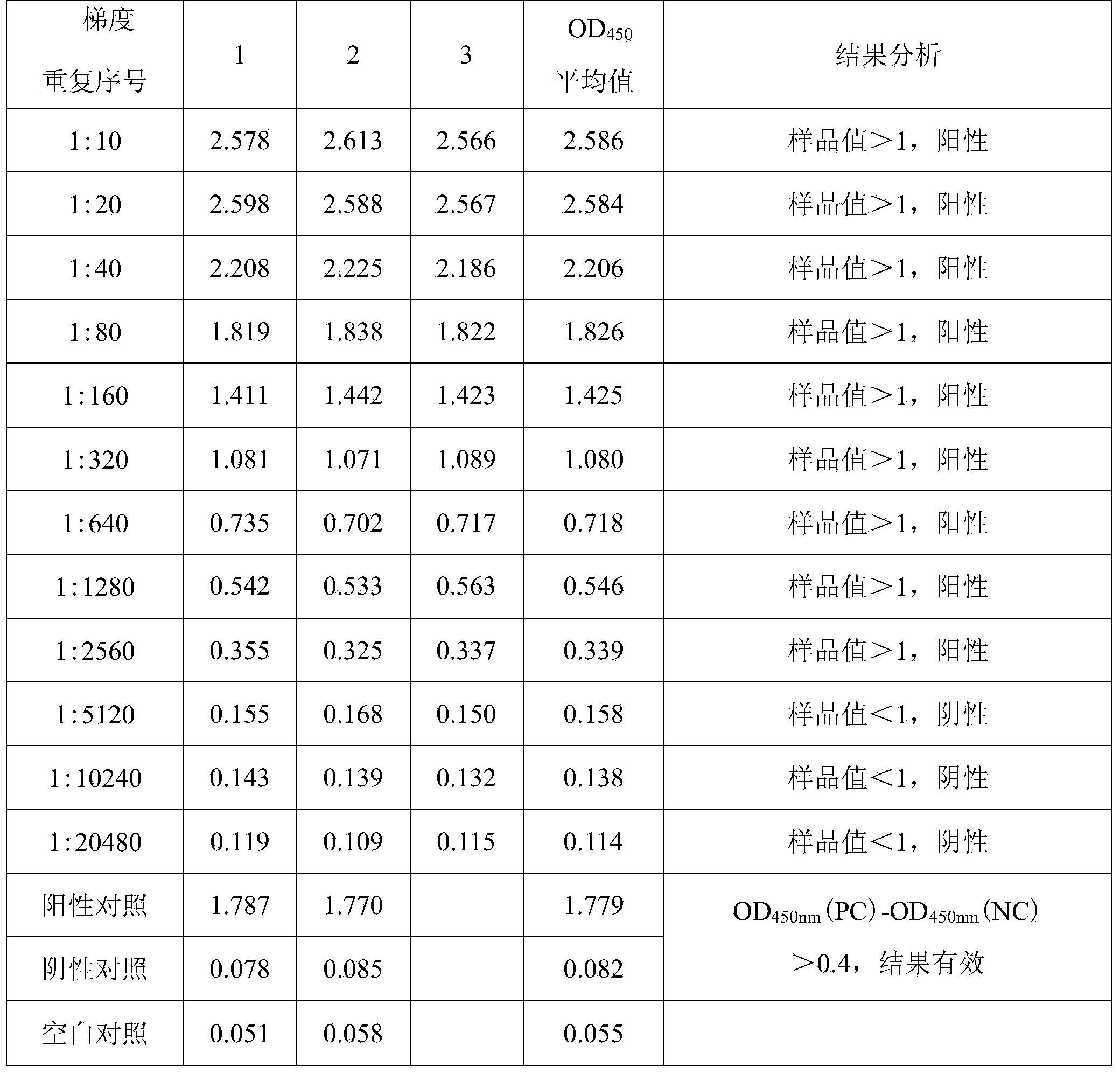
Image
Examples
Embodiment 1
[0045] The preparation of embodiment 1 swine fever virus Erns and E2 recombinant protein
[0046] (1) According to the complete genome sequence of CSFV Shimen strain in NCBI GeneBank (Accession No. AF333000.1), the complete sequence of envelope protein Erns of CSFV Shimen strain and the B / C region and D / A region of E2 main antigen region The gene sequence is used as a template to design synthetic primers, and the two primers are:
[0047] Erns (F): 5'-CGGGATCCGAAAATATAACTCAGTGGAACCTGA-3',
[0048] Erns(R): 5'-CGCTCGAGGGCATAGGCACCAAACCAG-3',
[0049] E2(F): 5'-CGGGATCCCGTCTAGCCTGCAAGGAAG-3',
[0050] E2(R): 5'-CGCTCGAGTAGATCTTCATTTTCCACTGTGG-3'.
[0051] Wherein, the underlined part is the restriction restriction site that introduces, and Erns (F) is CSFV-Erns gene upstream amplification primer, and the restriction restriction restriction site that introduces is BamHI, and Erns (R) is CSFV-Erns gene downstream amplification primer, introduces The enzyme cutting site is XhoI...
Embodiment 2
[0061] Example 2 Preparation and biotin labeling of polyclonal antibody against CSFV-Erns protein (CSFV-Erns) and polyclonal antibody against CSFV-E2 protein (CSFV-E2)
[0062] (1) The recombinant CSFV-Erns and CSFV-E2 proteins purified in Example 1 were used to immunize the experimental rabbits respectively, and blood was collected 7 days after the third immunization to detect the Elisa titer. After the rabbit serum titer reached 1:100,000, shock immunization was carried out , collect rabbit serum after 7 days;
[0063] (2) Recombinant CSFV-Erns and CSFV-E2 proteins were coupled to CNBr-activated Sepharose4B beads (purchased from GE) to prepare antigen affinity purification columns;
[0064] (3) Purify the rabbit serum prepared in step (1) with the affinity purification column prepared in step (2) to obtain rabbit anti-CSFV-Erns polyclonal antibody and rabbit anti-CSFV-Erns polyclonal antibody;
[0065] (4) The rabbit anti-CSFV-Erns polyclonal antibody and rabbit anti-CSFV-E...
Embodiment 3
[0069] Example 3 Preparation of Anti-CSFV Erns Protein (CSFV-Erns) Monoclonal Antibody and Anti-CSFV E2 Protein (CSFV-E2) Monoclonal Antibody
[0070] (1) Immunize Balb / c mice with recombinant CSFV-Erns and CSFV-E2 proteins purified in Example 1. After three immunizations, take mouse splenocytes and mouse myeloma cells SP2 with an Elisa titer of 1:10000 / 0 fusion, HAT medium 37 ℃, saturated humidity, 5% CO 2 Culture confluent cells.
[0071] (2) The colonies were observed on the fourth day after fusion, and when the colonies grew to about 1 / 6 of the bottom of the well, half of the HT medium was replaced. The next day, recombinant CSFV-Erns and CSFV-E2 protein-coated plates were used for indirect Elisa detection, and selection Positive wells were used for subcloning.
[0072] (3) Subcloning: Subcloning was carried out by conventional limiting dilution method. On the 5th day, the subcloning plate was coated with CSFV positive serum 1:1000 for indirect Elisa detection, and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com