Recombinant lentiviral vector for expression of a flaviviridae protein and applications thereof as a vaccine
A technology of recombining lentivirus and flavivirus, which is applied in the direction of virus/bacteriophage, the use of vectors to introduce foreign genetic material, viruses, etc., can solve the problem of reduced effectiveness of viral protein protection, and achieve the effect of increasing immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] Example 1 : Preparation of TRIPΔU3.CMV-sE (WNV) recombinant vector
[0152] 1) Construction of pTRIPΔU3.CMV-sE (WNV) vector plasmid
[0153] Polymerase chain reaction (PCR) was used to amplify the 967th to 2292th nucleotide sequence of the genome representing West Nile virus IS-98-ST1 strain (the sequence of patent application FR 01 04599 and Genbank accession number AF481864) and the corresponding polyprotein The cDNA of amino acids 291 to 732 (application FR 01 04599 and Genbank accession number AAL87234), the sense primers used are:
[0154] 5'-TAT CGTACG ATGAGAGTTGTGTTTGTCGTGCTA-3' (SEQ ID NO.18), containing the BsiW I site indicated by the underline; the antisense primer is:
[0155] 5'-ATA GCGCGC TTAGACAGCCCTTCCCAACTGA-3' (SEQ ID NO. 19), containing the underlined BssH II site. This cDNA corresponds to the sequence SEQ ID NO.16 in the appendix sequence listing, and the boundary is the BsiW I site at the 5' position and the BssH II site at the 3' position. ...
Embodiment 2
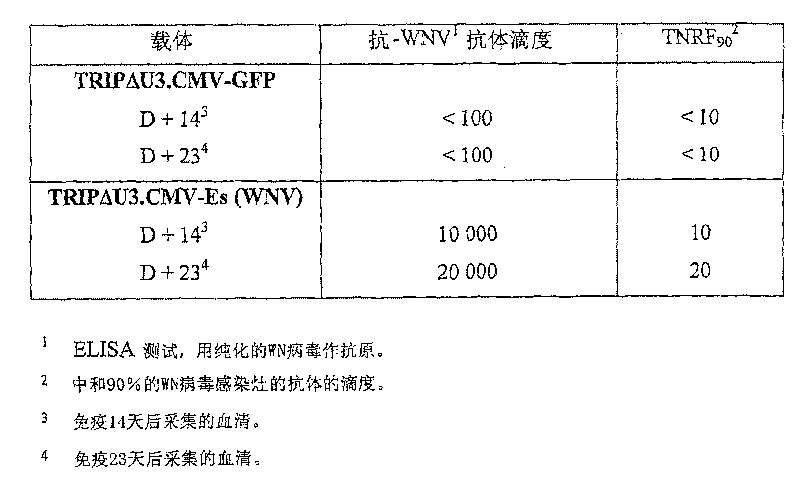
[0191] Example 2 : Analysis of the immunogenicity of TRIPΔU3.CMV-sE vector in BALB / c mice
[0192] 1) Materials and methods
[0193] 1.1) Immunization / Vaccination Program
[0194] 6-week-old BALB / c mice (2 groups, 6 mice each; Janvier breeding colony). Animals were given a single vaccine injection.
[0195] The control group was 1 μg TRIPΔU3.CMV-GFP vector particles prepared in a similar manner to TRIPΔU3.CMV-sE (WNV) vector particles under the same conditions (2 groups, 3 mice in each group) or only DPBS buffer (2 groups, 3 mice per group) were inoculated.
[0196] 14 days after vaccination (D 14 ) and 23 days (D23) later, mouse sera were taken and heat-inactivated at 56°C for 30 minutes before antibody responses were measured.
[0197] 1.2) West Nile virus strains, purification and titration
[0198] The West Nile virus strain used was the IS-98-ST1 strain, described in patent application FR 01 04599; it was produced on Aedes cells (AP61 line) and according to t...
Embodiment 3
[0217] Example 3 : Analysis of the protective ability of TRIPΔU3.CMV-sE(WNV) vector in BALB / c mice
[0218] In a mouse model of WNV-associated encephalitis, the protective effect of anti-E protein antibodies produced after immunization of mice with TRIPΔU3.CMV-sE (WNV) carrier particles was tested (Deubel et al., Ann.N.Y.Acad.Sci., 2001, 951, 195-206; Mashimo et al., 2002, previously cited; International Patent Application WO 02 / 081511; Ceccaldi et al., FEMS Microbiol. Lett., 2004, 233, 1-6). Therefore, by intraperitoneal injection of 10LD 50 (Lethal dose for 50% of mice) or 100LD 50 The highly neuroinvasive and neurotoxic West Nile virus IS-98-ST1 strain challenged immunized mice.
[0219] More precisely, two challenge protocols were used: (i) a first group of 6 mice immunized as described in Example 2 received 10 LD 15 days after vaccination 50 IS-98-ST1 strain virus; (ii) the second group of 6 mice immunized as described in Example 2 received 100LD after 30 days (D30) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com