Pig atypical pestivirus RT-PCR detection specific primer, kit and detection method
A type of atypical pestivirus and detection kit technology, applied in the field of animal virology and molecular biology, can solve the problems of poor applicability, rapid gene sequence variation, large gene sequence differences, etc., to achieve fast detection, simple operation, The effect of high specificity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 A kind of porcine SARS virus PCR detection primer, detection kit and detection method
[0035] 1, a kind of porcine SARS virus PCR detection primer
[0036] According to the strain sequence of porcine SARS virus registered by NCBI, accession numbers: KU194229, KX929062, LT594521, KU041638, KU041639, KU041637, KX929063, KX929069 and other information provided, using DNAMAN6.0 for comparative analysis, the following PCR was designed The primers:
[0037] Upstream primer F: ctcacyagtgatgggtggga (SEQ ID NO: 1), wherein, y represents: c or t;
[0038] Downstream primer R: cctatyttcttcatgaayaccatggc (SEQ ID NO: 2), wherein, y represents: c or t;
[0039] Using the above primers for PCR amplification can identify porcine atypical fever virus quickly and accurately.
[0040] 2. A porcine SARS virus PCR detection kit
[0041] A porcine atypical fever virus detection kit, comprising the above-mentioned porcine atypical fever virus PCR primers SEQID NO: 1 and ...
Embodiment 2
[0060] Example 2 specificity test
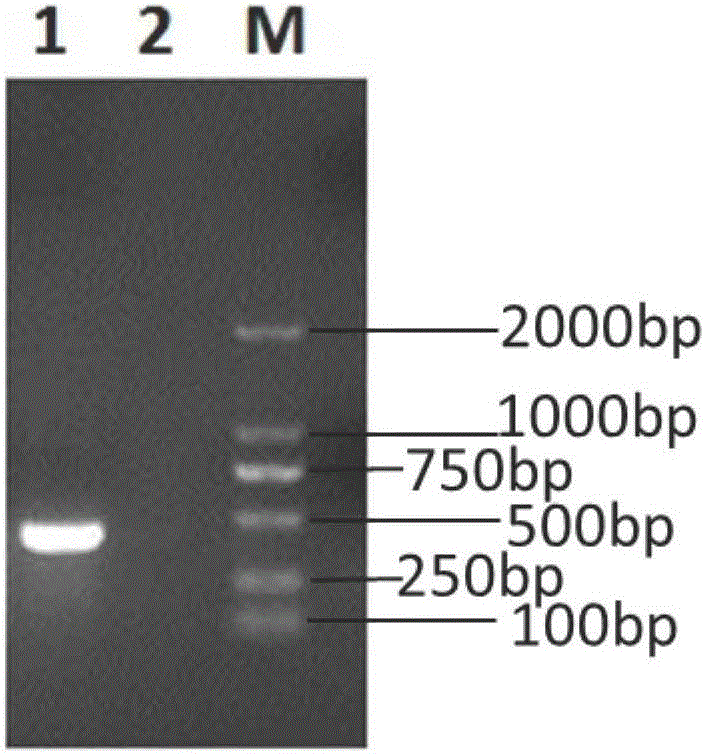
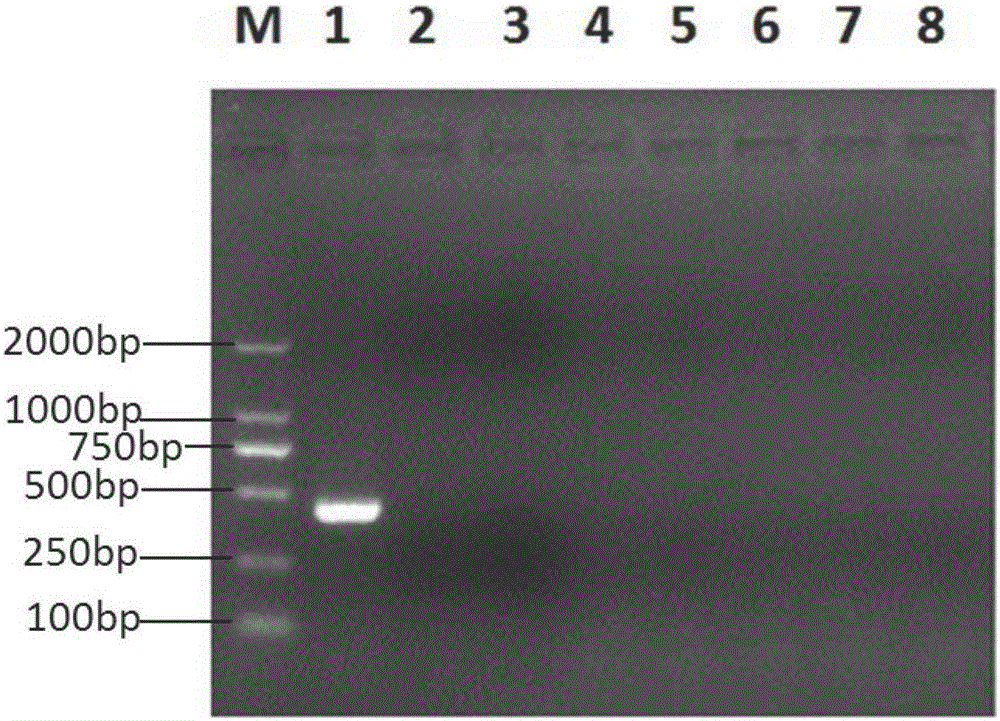
[0061] Serum sample RNA with positive detection is used as positive control, and sterile distilled water is used as negative negative control, according to embodiment 1 step (2), (3) amplification respectively, do electrophoresis identification by step (4) method, the result is as follows figure 1 shown.
[0062] Serum samples tested positive were used as positive controls, culture medium of porcine transmissible gastroenteritis virus, porcine rotavirus, porcine epidemic diarrhea virus, bovine viral diarrhea virus, swine fever virus, porcine reproductive and respiratory syndrome virus Carry out specificity detection, use the method of embodiment 1 and primer, the result does not all amplify except positive control, and positive disease material has band respectively at 439bp place through PCR amplification (see figure 2 ).
Embodiment 3
[0063] Embodiment 3 Sensitivity test
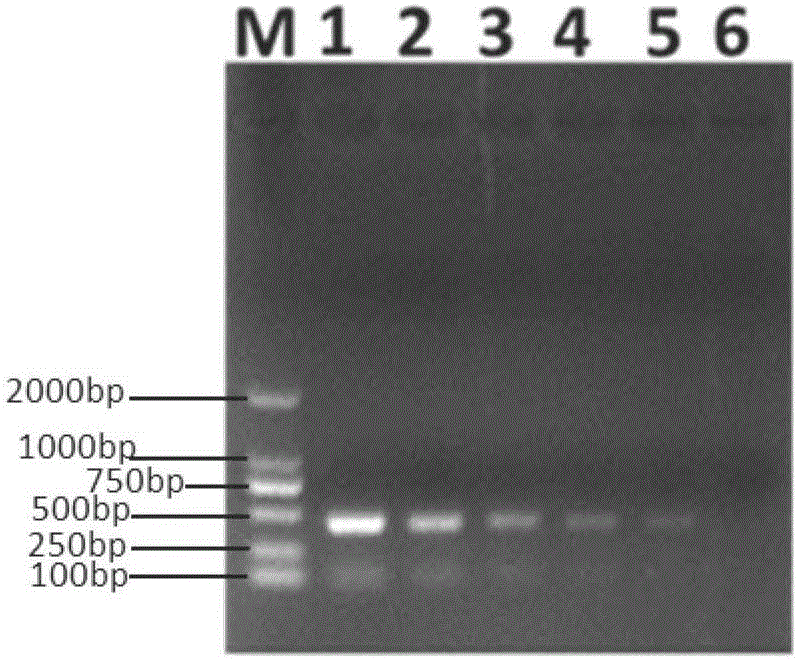
[0064] RNase Free H is used for the RNA extracted from the serum sample in Example 1 2 O made 10-fold serial dilution, the RNA content was 1.22μg / μL, 0.122μg / μL, 0.0122μg / μL, 1.22ng / μL, 0.122ng / μL, 0.0122ng / μL as a template, each dilution was taken 8 μL was used as a template, detected according to the method in Example 1, and the positive band was observed, and the sensitivity was calculated based on the highest dilution of the template amount used for the positive expected band, and the result showed that the minimum detection amount was 0.976ng, that is, 0.122ng / μL gradient. (See image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com