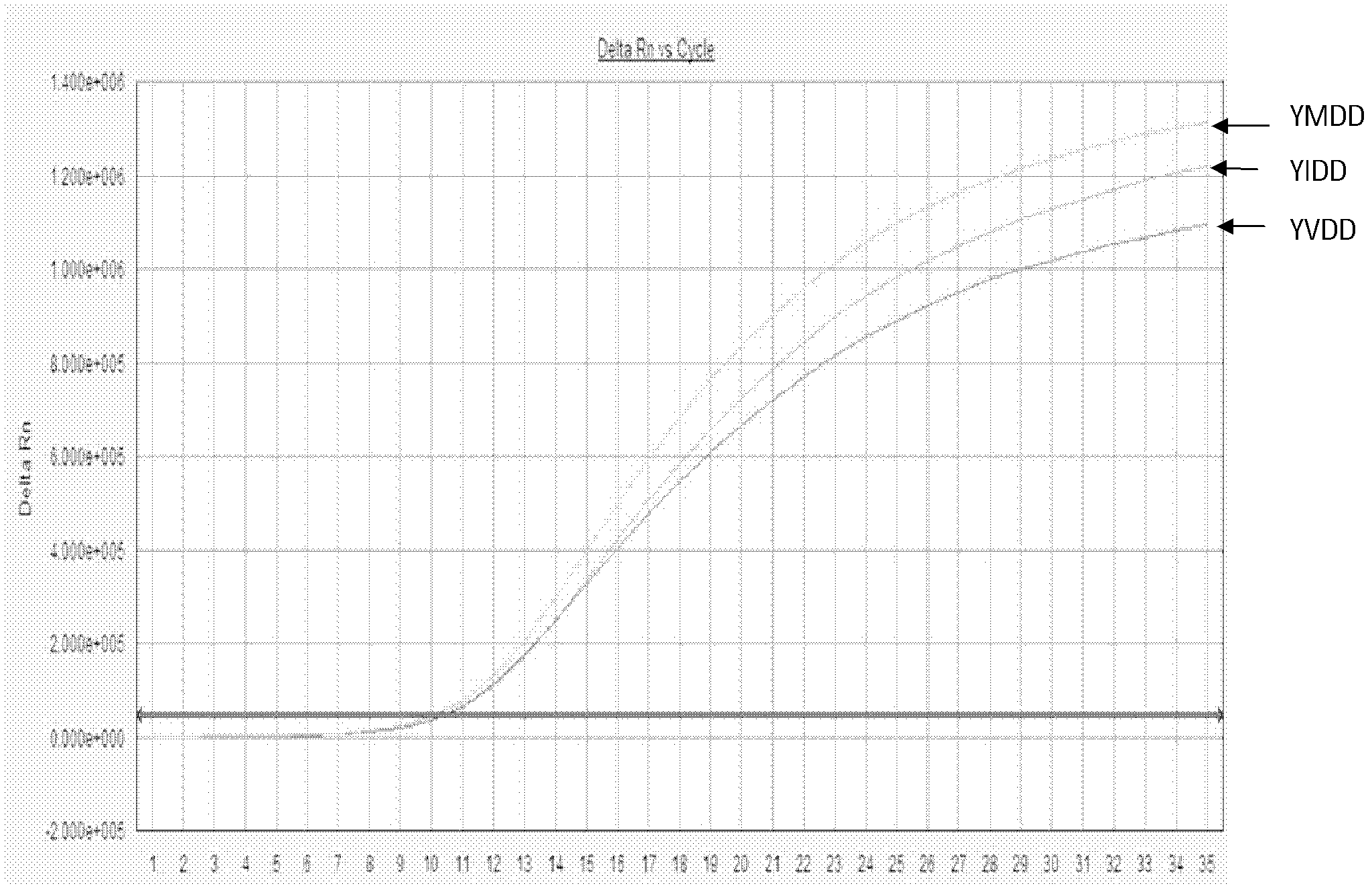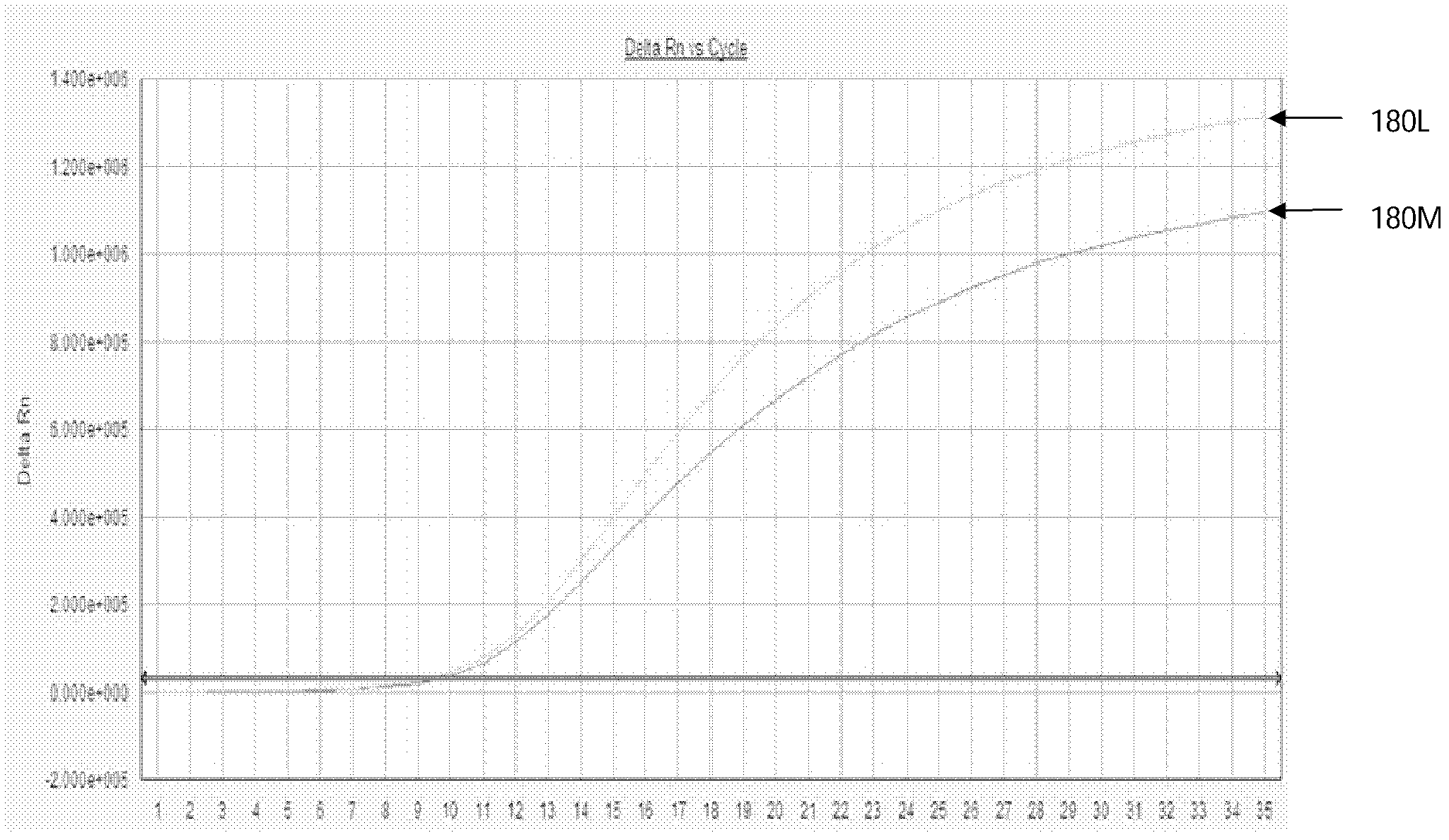Hepatitis B virus lamivudine resistant RNA quantitative detection primers and probes
A technology of hepatitis B virus and lamivudine, which is applied to the determination/testing of microorganisms, microorganisms, methods based on microorganisms, etc., can solve problems such as detection failures and false positives, and achieve reliable results, easy operation, and automation high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Method for detecting hepatitis B virus lamivudine drug-resistant nucleic acid on ABI7300 fluorescent quantitative PCR instrument with kit of the present invention
[0091] (1) Collect samples: collect human serum samples;
[0092] a. Use a sterile syringe to extract 1ml of venous blood from the subject, inject it into a sterile 1.5ml centrifuge tube, let it stand at room temperature for 2 hours, transfer it to 4°C for 1 hour, centrifuge at 8000rpm for 5 minutes, and draw 200μL of supernatant (note: Do not inhale red blood cells), transfer to another sterile 1.5ml centrifuge tube, which is the serum sample.
[0093] b. Specimen storage and transportation: If the specimen is not tested immediately, it should be stored at -20°C and avoid repeated freezing and thawing. Long-distance transportation of specimens should use 0 ℃ curling.
[0094] (2) Sample treatment: Take 100 μL of the serum to be tested, add an equal amount of 15% solution A, mix well, centrifuge at 13000 r...
Embodiment 2
[0112] According to the method of Example 1, the kit of the present invention is used to quantitatively detect the hepatitis B virus lamivudine drug-resistant nucleic acid of the unknown sample. The 60 unknown samples came from the hepatitis B virus samples to be tested for drug resistance in XX hospital patients.
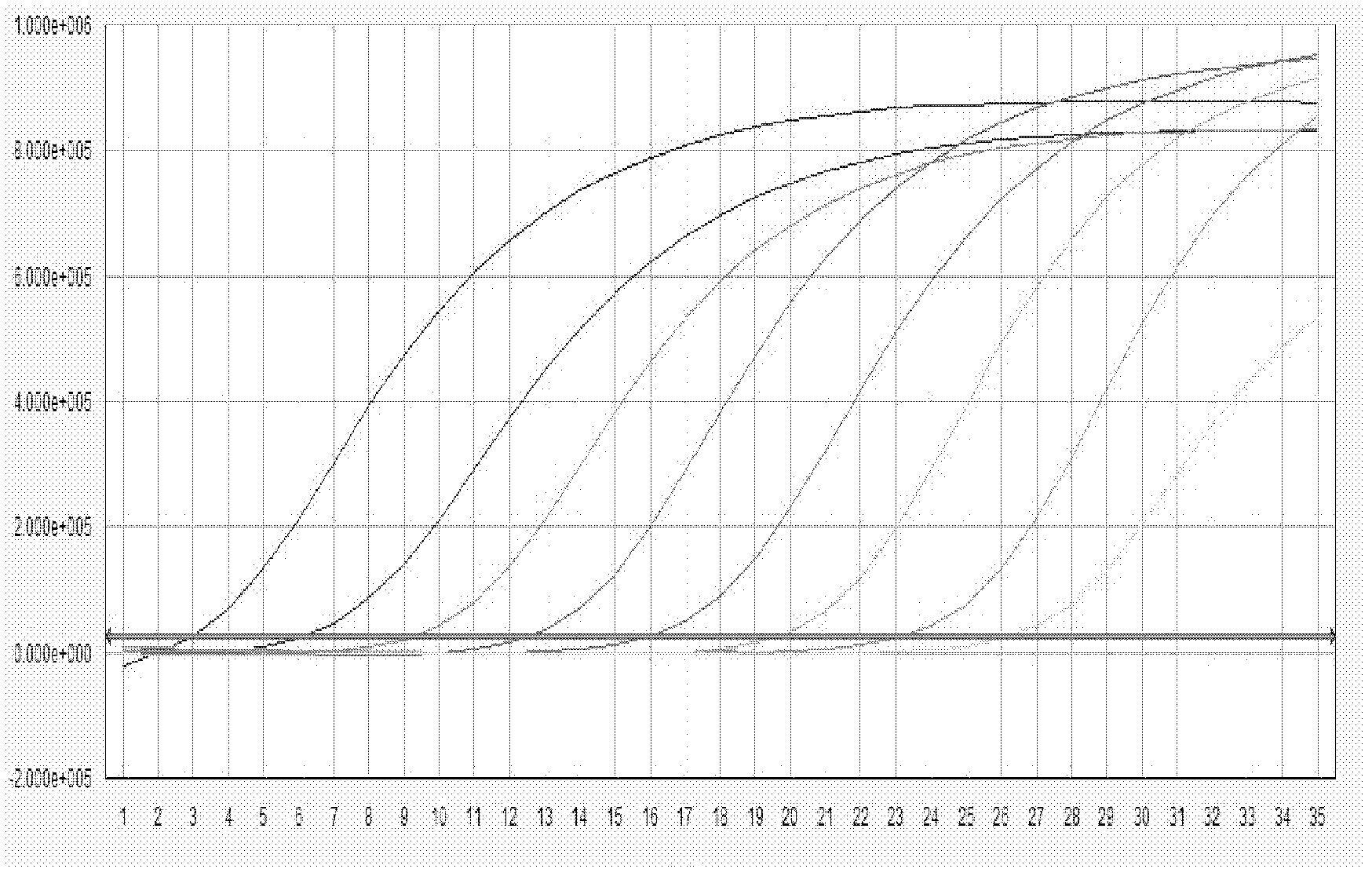
[0113] The results of the YMDD site probe in Example 2 of the present invention are respectively to the results of the sample fluorescent quantitative PCR test as shown in Figure 5, wherein, A, B: respectively represent the amplification curve and standard curve of rt204M (wild strain); C, D : Represent the amplification curve and standard curve of rt204I (mutant strain) respectively; E, F: Represent the amplification curve and standard curve of rt204V (mutant strain) respectively; As can be seen from the figure, the curve is smooth S-shaped, and the amplification effect better. The results of the rt180 site probes on the sample fluorescent quantitative PCR test a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com