Adenovirus vectors specific for cells expressing androgen receptor and methods of use thereof
a technology of adenovirus and adenovirus, which is applied in the field of cell-specific replication of adenovirus vectors, and the replication of adenoviruses that are replication-competent, can solve the problems of urinary incontinence, hyperplastic prostate growth, and immense cost of treating these three diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Adenovirus Vectors Containing an Earl) Gene Under Control of a Transcriptional Element Derived from a Probasin Transcriptional Response Element (PB-TRE)
1. A. The Probasin Transcriptional Response Element (PB-TRE)
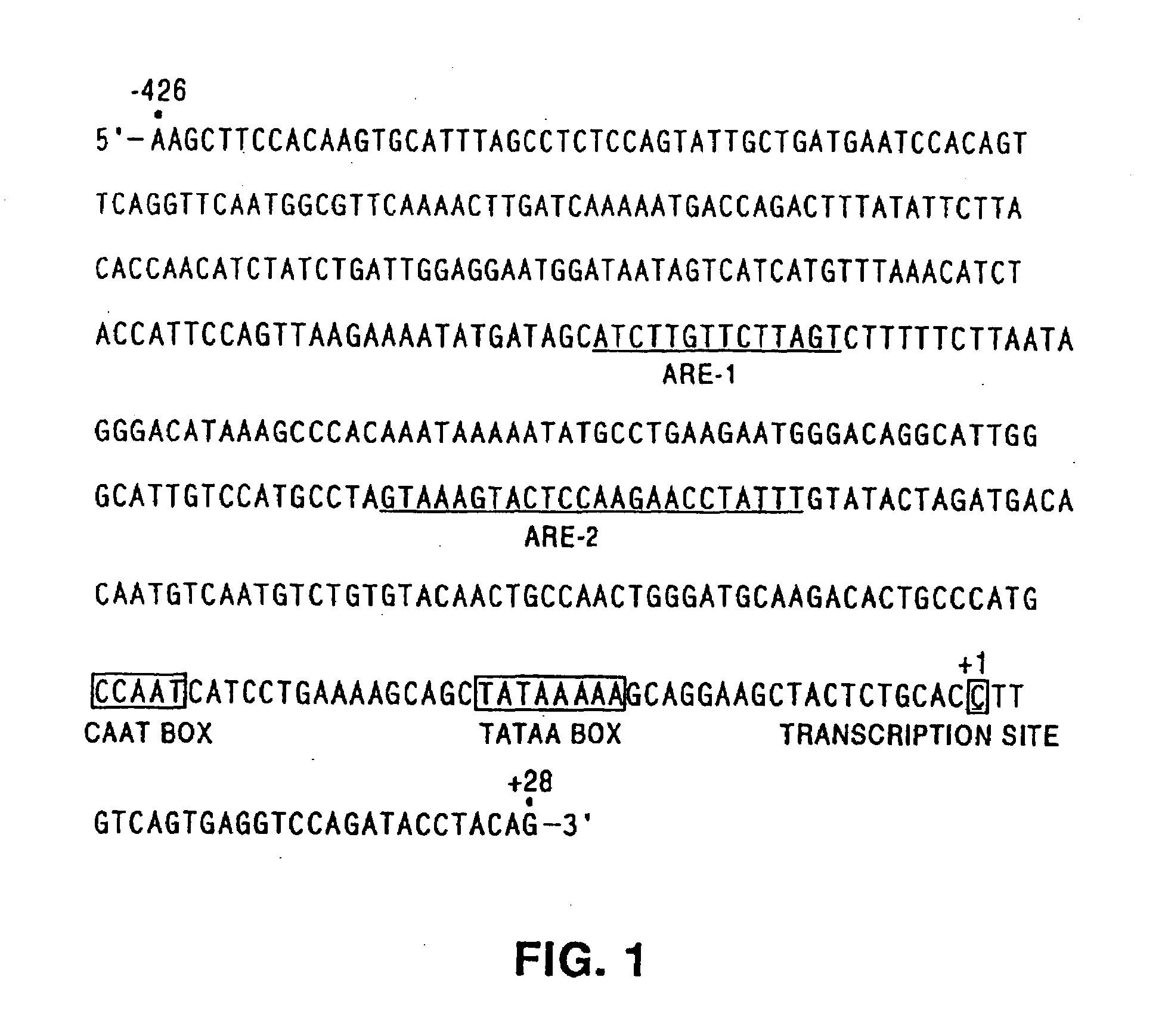
[0173]The 454 nucleotide fragment (nt about −426 to about +28) of the rat PB-TRE, which contains two androgen response elements (ARE sites), a CAAT box and a TATAA box (FIG. 1, SEQ ID NO:1), %% as amplified by polymerase chain reaction (PCR) using rat genomic DNA as template and the synthetic oligonucleotides:
42.2.1 (SEQ ID NO: 4):5′-GATCACCGGTAAGCTTCCACAAGTGCATTTAGCC-3′,PinAI site underlined,and42.2.2 (SEQ ID NO: 5):5′-GATCACCGGTCTGTAGGTATCTGGACCTCACTG-3′,or oligonucleotides42.2.3 (SEQ ID NO: 6):5′-GATCCGGCCGAAGCTTCCACAAGTGCATTTAGCC-3′,EagI site underlined,and42.2.4 (SEQ ID NO: 7):5′-GATCCGGCCGCTGTAGGTATCTGGACCTCACTG-3′.
The oligonucleotides created a unique PinAI (AgeI) site (A / CCGGT) or EagI site (C / GGCCG) at both ends of the PCR fragments. The PCR fragments were ligated i...
example 2
Testing a Putative PB-TRE
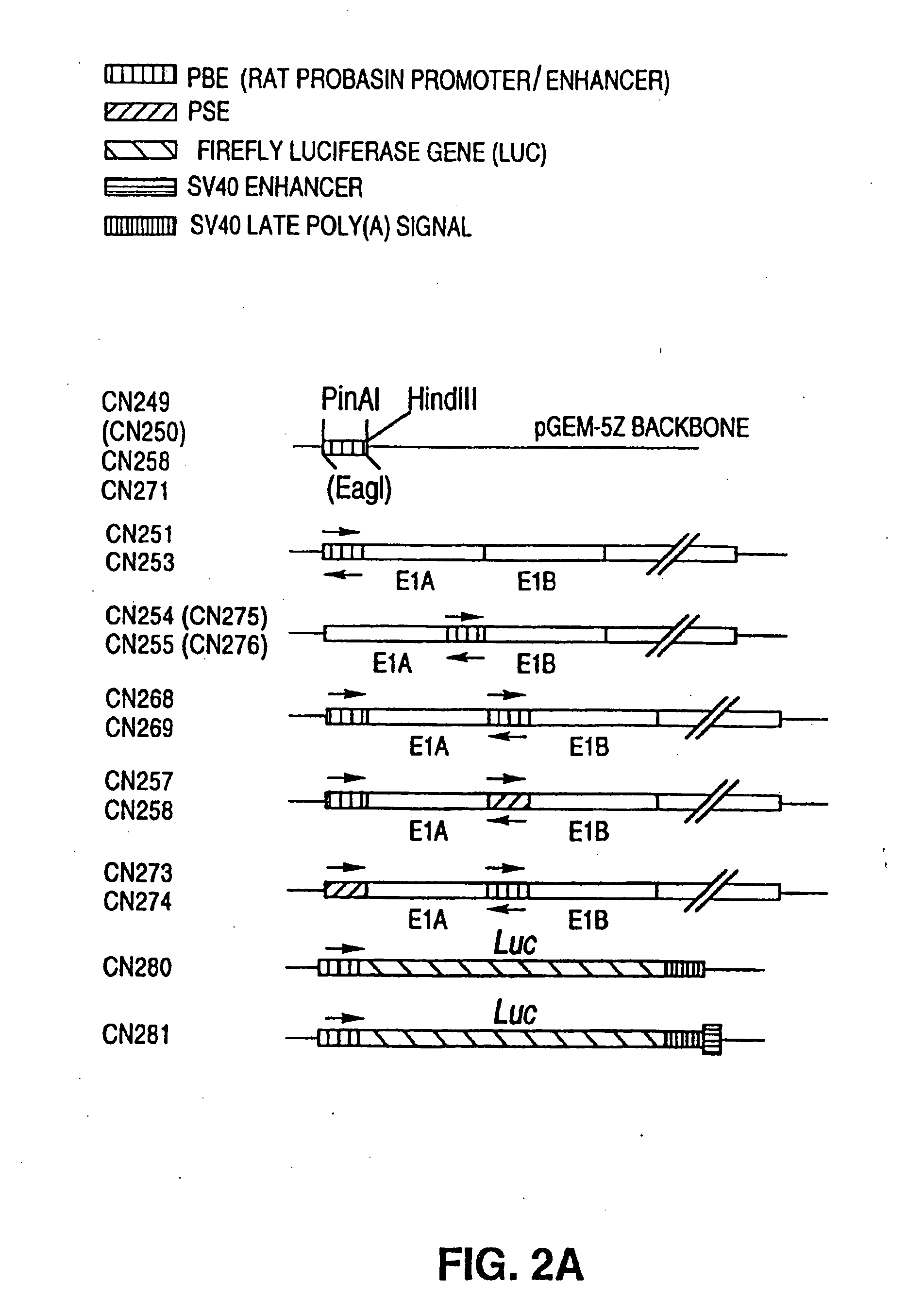
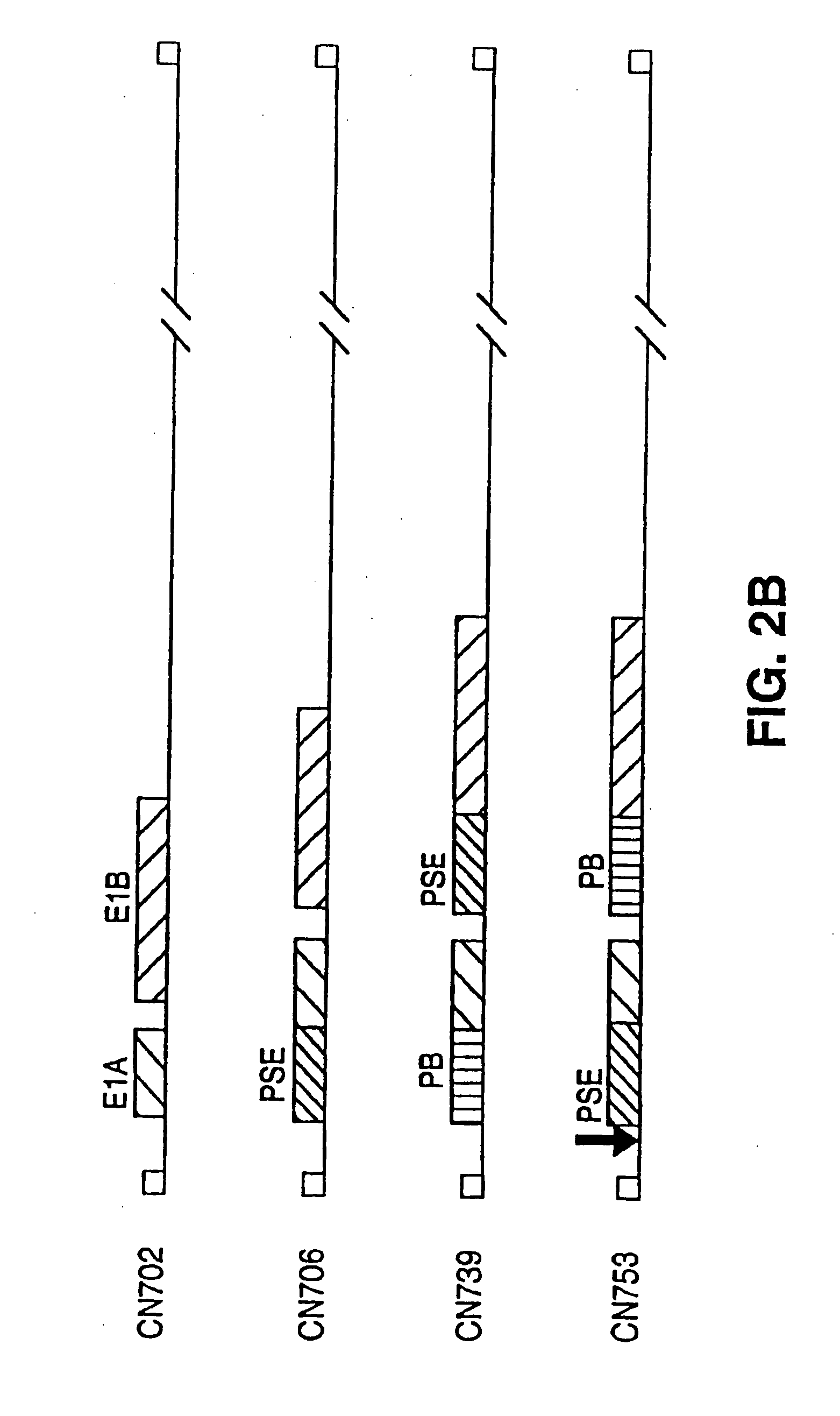
[0212]Plasmids similar to a reporter plasmid, such as CN280 described above, may be used to test the prostate-specificity of putative prostate-specific TREs (PS-TREs) or PS-TRE variants such as PB-TRE variants. The PS-TRE to be tested may have mutations such as deletions or insertions between binding sites known to be important in PS-TRE activity (e.g., are sites, etc.) or base substitutions in these sites themselves. For example, some variants in the AREs (androgen receptor binding sites) of PB-TREs are known and described above and in the literature. Rennie et al. (1993) Mol. Endocrinol. 7:23-36. Additional variant PB-TREs may comprise, for example, mutation(s) between a PB-TRE promoter and a PB-TRE enhancer (including deletions, substitutions and additions); a combination of a non-prostate specific promoter and a PB-TRE enhancer; a combination of a non-prostate-specific promoter and a PSA-TRE enhancer; the rearrangement of segments of the enhancer and pro...
example 3
Testing the Cytotoxic Ability of Adenovirus Vectors on Prostate Carcinoma Cells and Tumor Xenografts
[0215]3.A. Experimental Strategy
[0216]An especially useful objective in the development of prostate-specific adenoviral vectors is to treat patients with prostate carcinoma. The strategy is to develop a prostate tissue-specific targeting pharmaceutical which could selectively kill a certain type of tumor cells (such as prostatic neoplasia) while leaving their non-cancerous neighbors unharmed. A ‘smart bomb’ virus, in which key gene expression is restricted by a tissue-specific regulatory element incorporated into its chromosome, meets this requirement. As stated previously, PB gene expression is exclusively in the prostate and is transcriptionally regulated by androgens. It has also been demonstrated that the minimal 454 nucleotides of PB-TRE is sufficient to target and restrict gene expression to prostate cells. This arrangement limits the expression of important early viral genes to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com