Methods and compositions for improved non-viral gene therapy
a non-viral gene therapy and composition technology, applied in the field of molecular biology and pharmacology, can solve the problems of limited viral vectors, difficult targeting, and relatively small capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
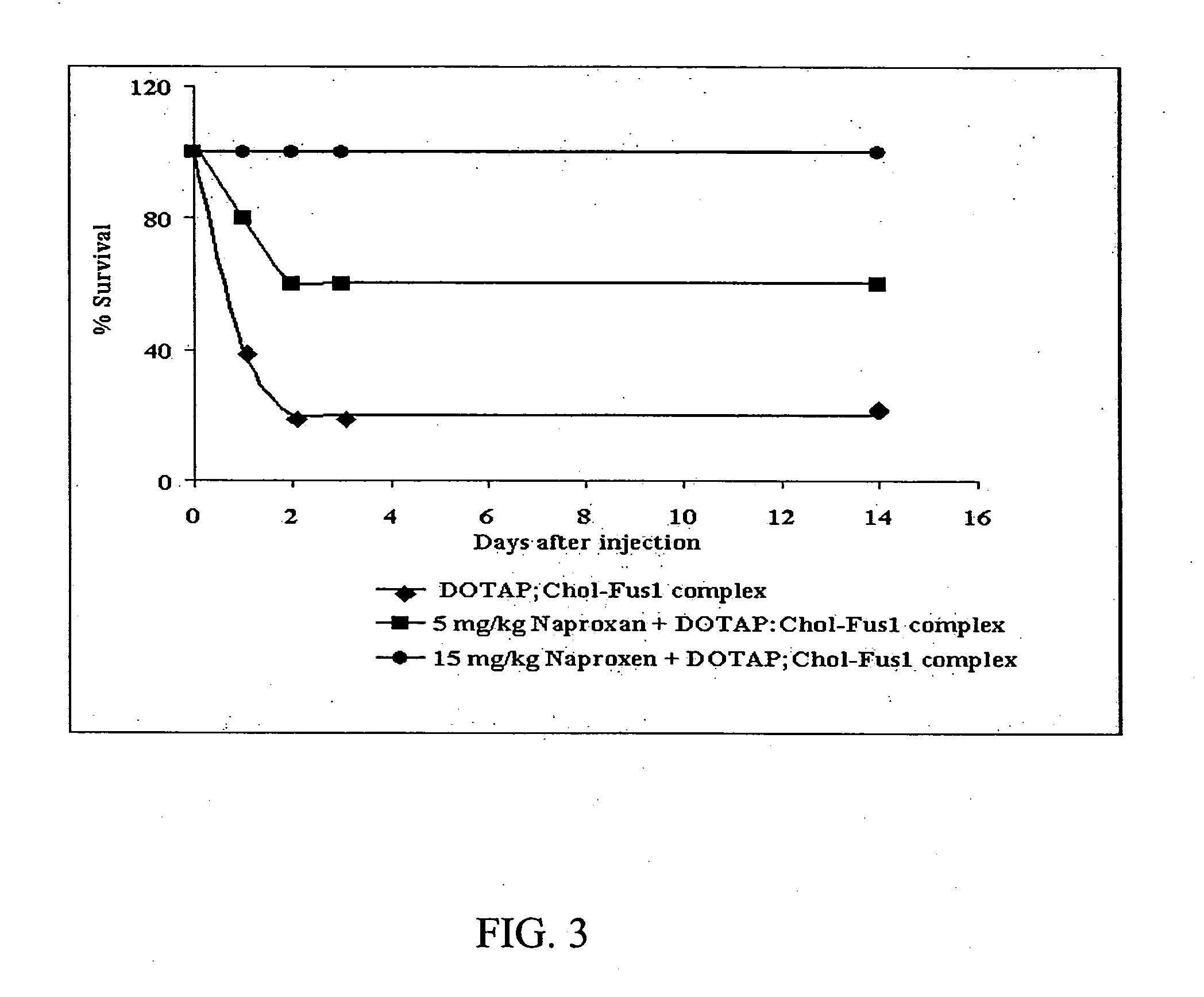
Effect of Naproxen on Transgene Expression In Vivo
Purpose
[0285] These in vivo studies were conducted to determine whether Naproxen can inhibit the DOTAP:Chol-DNA complex mediated transgene expression in vivo and thereby affect the therapeutic efficacy. These in vivo studies therefore utilized oral administration of Naproxen followed by intravenous DOTAP:Chol-FUS1 treatment in immunocompetent C3H mice for testing the inhibitory effects.
Materials and Methods
[0286] Animals. Female C3H mice (4-6 weeks old) were purchased from NCI (Fredericksburg, Md.) and housed in a pathogen free room in the Department of Veterinary Medicine and Surgery, M.D. Anderson Cancer Center, Houston, Tex.
[0287] Plasmid. The plasmid DNA used was pLJ-143, and the gene was FUS1. The plasmid concentration used was 10.20 mg / ml. The plasmid purification was proprietary, and the source of the plasmid DNA was Selective Genetics. All DNA preparations were stored at −70° C. Plasmid DNA used in the present study wa...
example 2
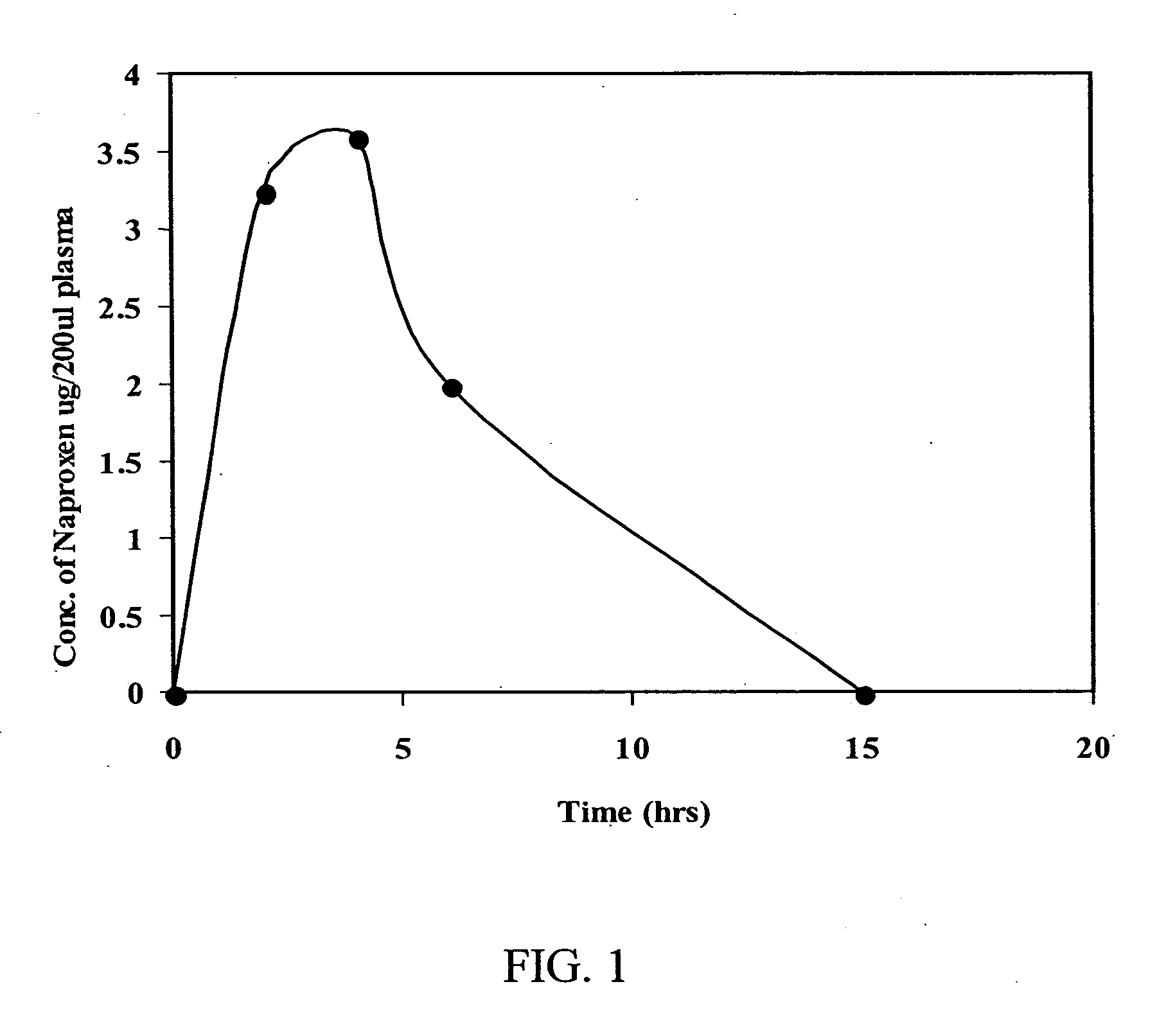
[0295] Analysis of Naproxen Levels in Blood
Purpose
[0296] These in vivo studies were conducted to determine the circulating levels of naproxen in the blood. These in vivo studies therefore utilized oral administration of naproxen in immunocompetent C3H mice.
Materials and Methods
[0297] Animals. Female C3H mice (4-6 weeks old) were purchased from the National Cancer Institute (Fredericksburg, Md.) and housed in a pathogen-free room in the Department of Veterinary Medicine and Surgery, M.D. Anderson Cancer Center.
[0298] Naproxen. Clinical grade naproxen was purchased from the pharmacy at M.D. Anderson Cancer Center.
[0299] Administration and analysis of Naproxen. Female C3H mice (n=12) were administered clinical grade naproxen orally in a volume of 100 μl to give a final concentration of 15 mg / kg. Animals were euthanized at 2, 4, 6, and 15 hours after treatment, and blood was collected. Blood samples were stored at −80° C. until all samples were collected. They were then submitte...
example 3
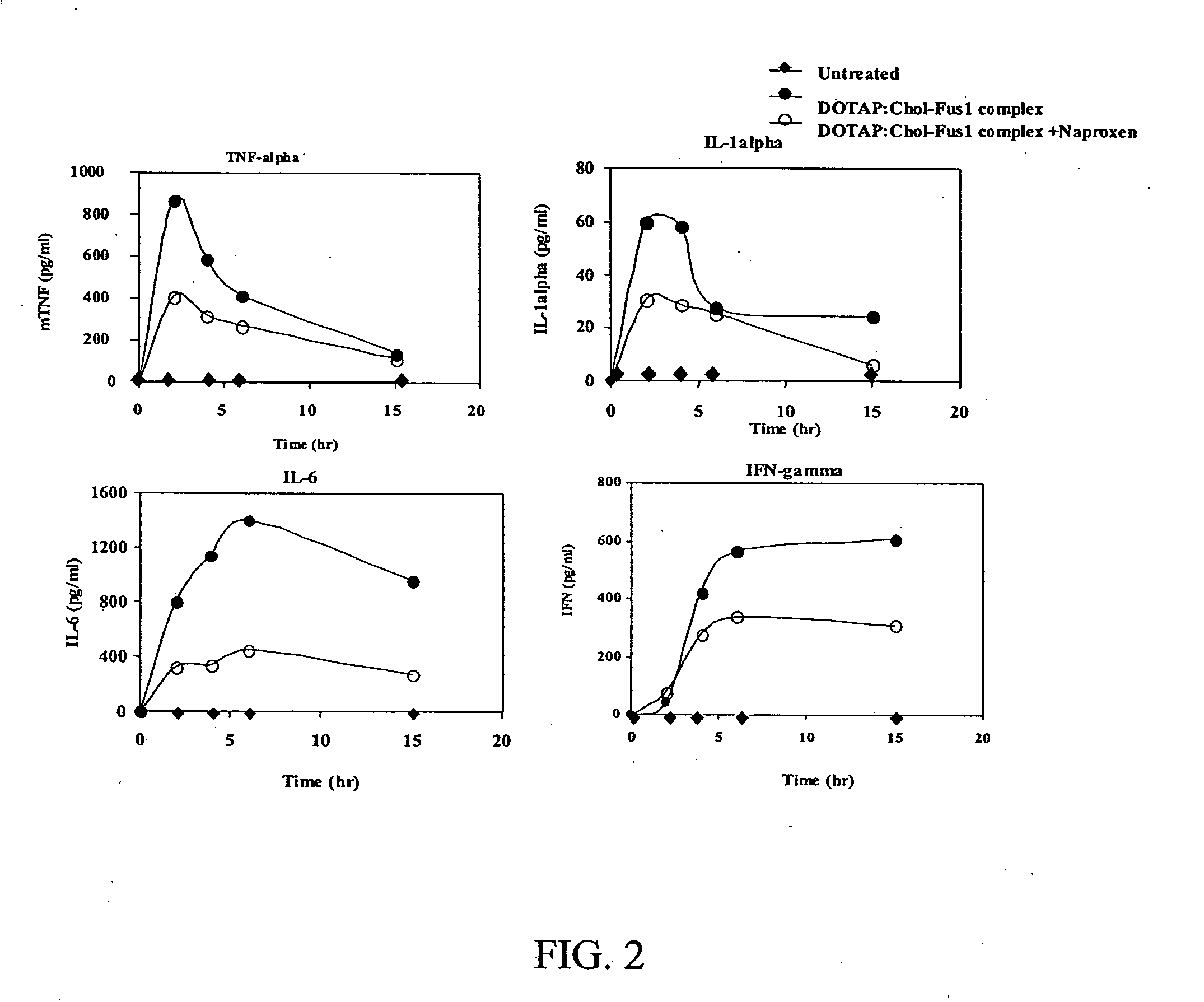
DOTAP:Chol-FUS1 Complex Suppresses Cytokine Production In Vivo
Purpose
[0301] These in vivo studies were conducted to determine whether naproxen can inhibit the DOTAP:Chol-DNA complex induced inflammatory response and thereby protect mice from toxicity. These in vivo studies therefore utilized oral administration of naproxen followed by intravenous DOTAP:Chol-FUS1 treatment in immunocompetent C3H mice for testing the protective effect.
Materials and Methods
[0302] Materials and methods pertaining to the animals, plasmid, liposome preparations, preparation of the DOTAP:Cholesterol-FUS1 complex are as described in Example 1.
[0303] Particle size analysis of DOTAP:Cholesterol-Fus 1 complex. The particle size of the DOTAP:Chol-FUS1 complex was determined using the N4-Coulter Particle Size analyzer (Beckman-Coulter). Briefly, 5 μl of the freshly prepared was diluted in 1 ml of water and particle size determined.
[0304] Spectrophotometric reading of DOTAP:Cholesterol-FUS1 complex at O.D...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com