Humanized rodents for testing therapeutic agents
A rodent and human antibody technology, applied in compound screening/testing, chemical instruments and methods, polypeptides containing positioning/targeting motifs, etc., can solve problems such as limiting safety and usefulness, and reducing usefulness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1048] Example 1: Genetic Engineering of Mice Containing Humanized Immunoglobulin Constant Regions
[1049] This example illustrates an exemplary method for constructing a series of targeting vectors for insertion into the genome of a non-human animal such as a rodent (eg, mouse). The methods described in this Example demonstrate the generation of non-human animals whose genomes comprise engineered immunoglobulin heavy chain constant regions and / or engineered immunoglobulin light chain (kappa or lambda) constant regions. In particular, this example demonstrates the construction of targeting vectors for engineering immunoglobulin heavy constant regions to allow non-human animals to express and / or produce antibodies comprising immunoglobulin heavy chains Having a human variable domain and a constant domain that is fully or partially human (eg, comprising a human portion and a rodent portion). It also demonstrates the construction of targeting vectors for engineering immunoglobu...
Embodiment 11
[1053] Example 1.1: Genetic Engineering of Mice Comprising Human Heavy Chain Variable Regions and Chimeric or Fully Human Heavy Chain Constant Regions
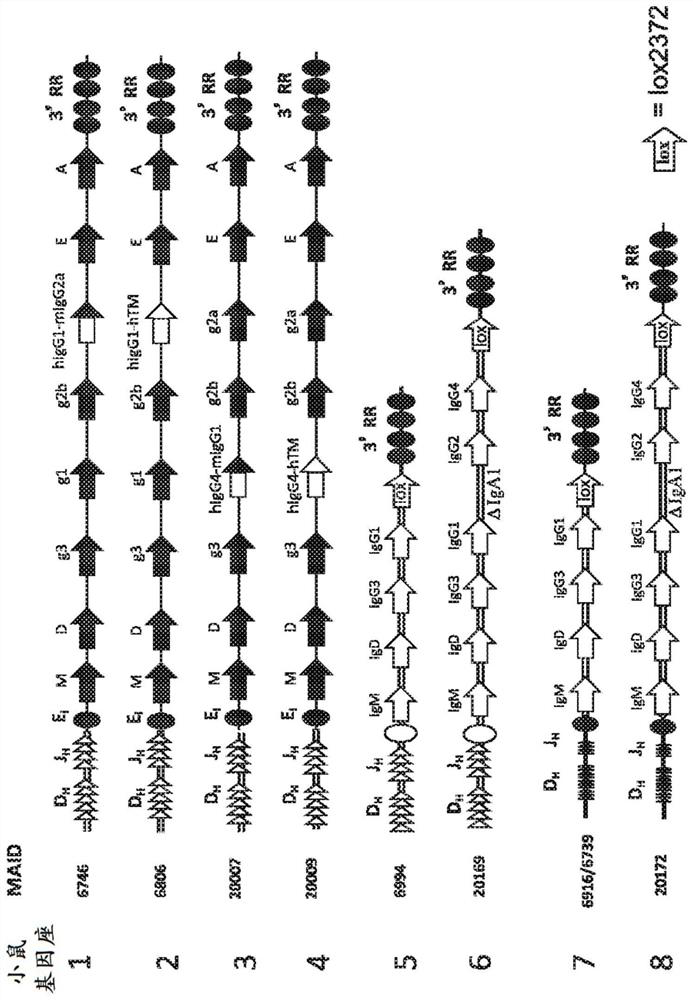
[1054] To generate rodents that can be used in human antibody testing (eg, pharmacokinetic and dosing studies of therapeutic antibodies), several methods have been implemented. In the first approach, a human heavy chain variable region and a chimeric (human C H 1-H-C H 2-C H 3 coding sequence and mouse M1-M2 coding sequence) or fully human (C H 1-H-C H 2-C H 3-M1-M2 coding sequence) heavy chain constant region mouse of the heavy chain constant region gene segment. Such mice can be used, for example, to test the relationship with immunoglobulin constant domains encoded by modified gene segments (i.e., the C of the constant domains). H 1-H-C H 2-C H Part 3) Isotype-matched therapeutic antibodies (eg, testing IgG1 antibodies in mice containing genetically engineered IgG1 constant domains).
[1055] In one example, a chim...
Embodiment 12
[1083] Example 1.2: Genetic Engineering of Mice Comprising Mouse Heavy Chain Variable Regions and Human Heavy Chain Constant Regions
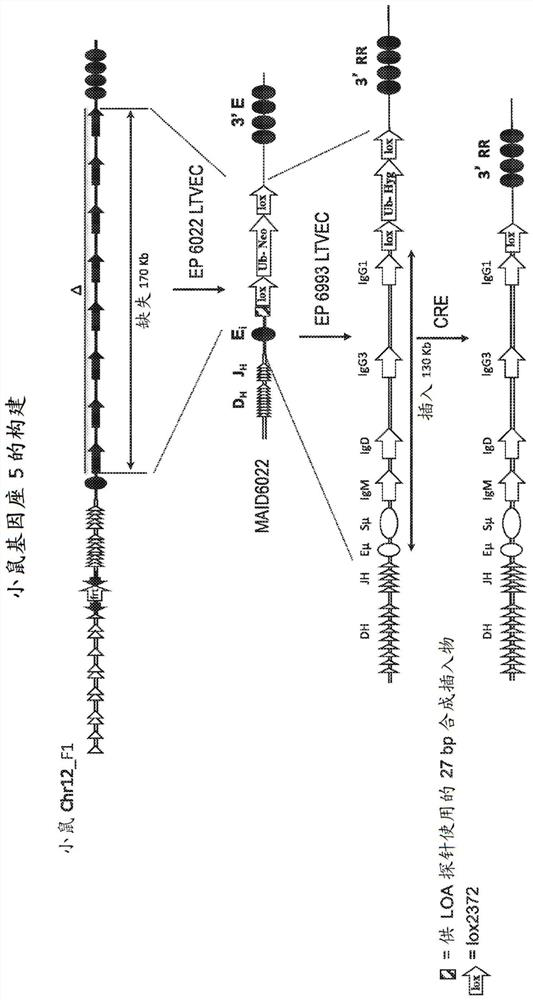
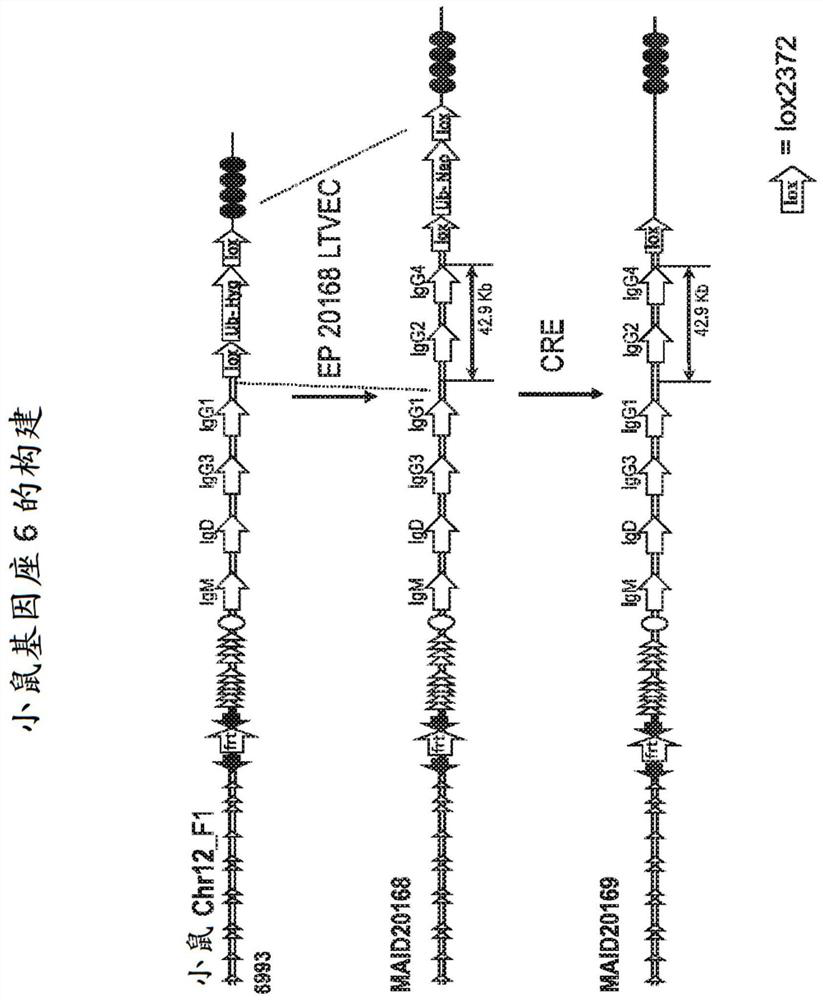
[1084] To generate constructs for targeting mouse ES cells comprising a mouse heavy-chain variable region and a human heavy-chain constant region, an intronic enhancer (Eμ) and a 3' regulatory region (3' RR) deletion constructs of the entire mouse IgH constant region (IgM, IgD, IgG3, IgGl, IgG2b, IgG2a, IgE and IgA).
[1085] Briefly, a 5.2 kb 5' mouse homology arm was amplified from BAC clone BMQ-451A16 (The Sanger Centre) by PCR and cloned into a plasmid with the R6K origin of replication and the lox2372-ub-neo cassette using isothermal assembly middle. The resulting plasmid contains from 5' to 3': 392bp R6K origin, unique I-CeuI site, 5.2kb region of the mouse IgH locus including DQ52, JH1-4 and Eμ, unique NotI site, lox2372-ub-neo box and a unique AscI site. This plasmid was digested and the I-CeuI-AscI fragment of the plasmid ligated in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com