Neonatal Fc receptor (FcRn)-binding polypeptide variants, dimeric Fc binding proteins and methods related thereto
An amino acid, charged technology, used in the preparation of polypeptides containing this mutation, identification of desired amino acid mutations, and preparation of the altered polypeptides, PCT application field, can solve the problems of circulating half-life antibodies that cannot be used as therapeutic agents, low bioavailability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0715]Example I-Preparation of modified antibodies with chimeric Fc region
[0716] In order to evaluate the binding of the human rabbit IgG chimeric construct, the amino acids that may affect the binding are first determined to be within 10 Ȧ of the interaction interface between the two interacting proteins. Based on the crystal structures of rat IgG2a and rat FcRn, the amino acids on rat IgG2a Fc located within 10 Ȧ of the interface between these two molecules were determined. Then the homology comparison of the rabbit IgG1 Fc and rat IgG1 Fc regions allows to determine which amino acids on the rabbit Fc region may be within 10 A of the interaction interface. The rat and human Fc region counterparts were then determined. The chimeric molecule is then constructed, including all mutants within the 10 Ȧ interface. Then each single amino acid was substituted into hFc separately, and analyzed to determine the contribution of each single component amino acid to the binding. A combinat...
Embodiment 2
[0724] Example 2-Identification of candidate residues by electrostatic optimization
[0725] In this example, a method for modifying the affinity of the constant region of an antibody to human FcRn is described. In order to obtain mutants whose Fc region has altered binding affinity to FcRn at acidic pH and neutral pH, we applied electrostatic charge optimization technology to a homologous model of human Fc that binds to human FcRn. The model of the human Fc / FcRn complex at acidic (6.0) pH and neutral (7.4) pH uses the MODELLER program (Accelrys, Inc., San Diego, CA) from the crystal structure of the rat Fc / FcRn complex (PDB) Code: 1I1A) was derived and energy minimization was performed in CHARMM (Accelrys, Inc., San Diego, CA). In the process of computational optimization, we use electrostatic charge optimization to determine the positions of Fc residues that can adjust the binding of Fc and FcRn at acidic pH and neutral pH (Lee and Tidor, J. Chem. Phys. 106: 8681-8690 , 1997; Ka...
Embodiment 4
[0752] Example 4: Construction of modified Fc polypeptide
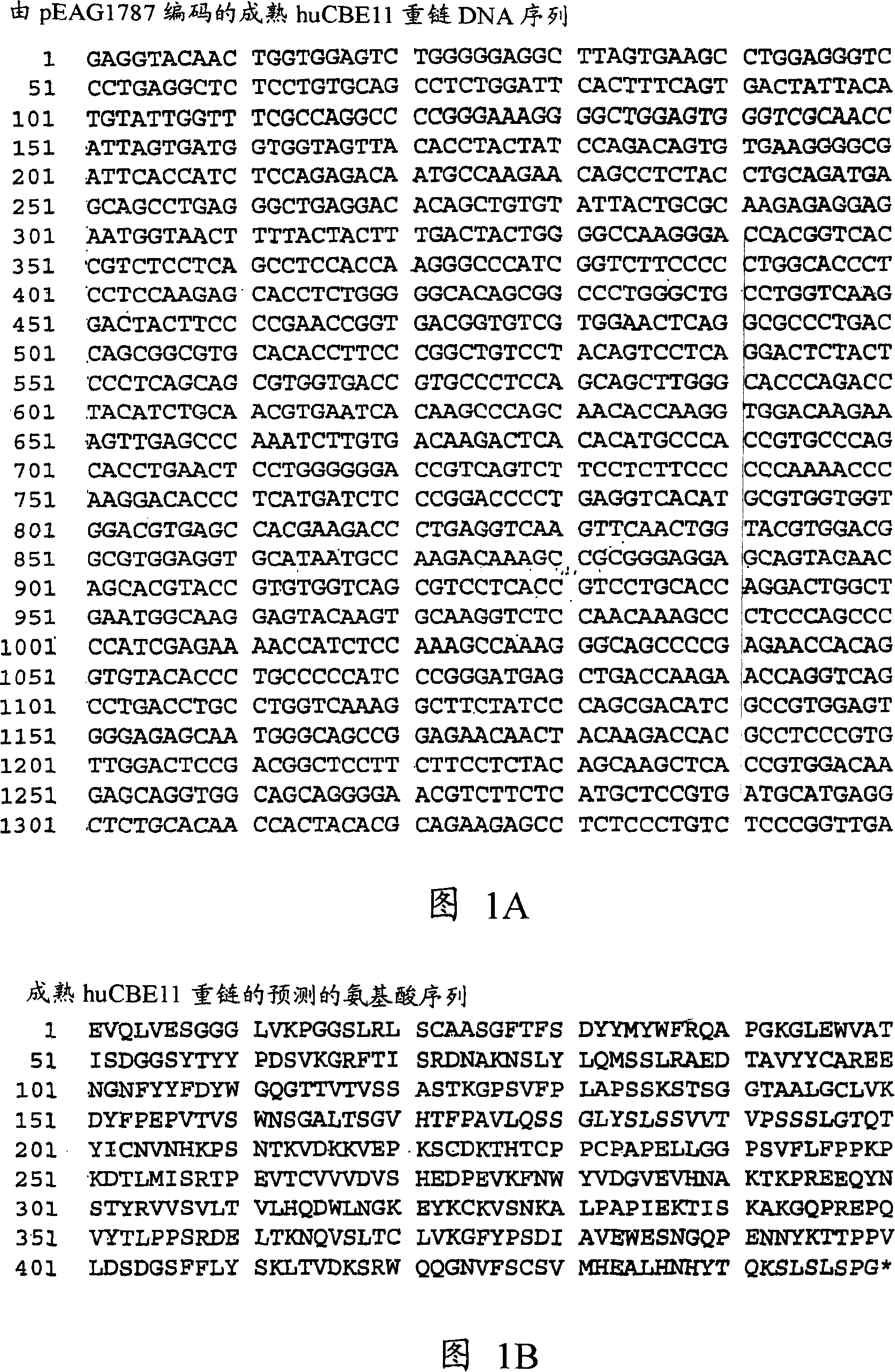
[0753] The changes predicted by the method of the present invention are introduced into the starting polypeptide encoding the heavy chain of the humanized IgG1 monoclonal antibody huCBE11. Figures 1A and 1B show the nucleotide sequence (SEQ ID NO: 3) and predicted amino acid sequence (SEQ ID NO: 4) of the heavy chain, respectively. Using standard recombinant DNA technology, site-directed mutagenesis was used to introduce mutations into the huCBE11 heavy chain carried on an expression vector called pEAG1787. The antibody variable region is the residues 1-120, and the human IgG1 constant region is the 121-449 residues. The C-terminal lysine residue of huIgG1 was genetically removed. For reference: the N-linked glycosylation site (EU residue number N297) in the above sequence is the 300th residue. Figure 2 shows the amino acid sequence of the Fc region of huCBE11 according to the EU numbering index.
[0754] HuCBE11 monoclon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com