Hybrid antibodies
A technology of antibody and receptor, applied in the field of hybrid IgE antibody
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0113] Functionally similar amino acids that can be exchanged by conservative substitutions are well known to those of ordinary skill in the art. The following six groups are examples of amino acids that are considered conservative substitutions for each other: 1) Alanine (A), Serine (S), Threonine (T); 2) Aspartic acid (D), glutamic acid ( E); 3) Asparagine (N), Glutamine (Q); 4) Arginine (R), Lysine (K); 5) Isoleucine (I), Leucine (L) ), methionine (M), valine (V); 6) phenylalanine (F), tyrosine (Y), tryptophan (W).
[0114] The domains described above (eg, one or more IgE and IgG constant domains) are typically present in the heavy chains of antibodies. In addition to one or more heavy chain sequences as described herein, a hybrid antibody may also comprise one or more light chains. For example, in one embodiment, a hybrid antibody may comprise a light chain sequence as defined by SEQ ID NO:35, or a fragment or variant thereof. Antibodies are usually composed of heavy an...
Embodiment 1-Fc
[0140] Example 1 - FcRn constructs
[0141] IgE variants were created in which point mutations were made in loops found in the Cε3 and Cε4 domains of IgE. These mutations replaced original amino acids with histidines at positions known to be involved in IgG-FcRn interactions. IgE antibodies are based on trastuzumab IgE, eg as Karagiannis et al. (2009) Cancer Immunol. Immunother. 58 (6): disclosed in 915-30.
[0142]Additional variant IgE antibodies were generated in which loops in the Cε3 and Cg4 domains of IgE were replaced by one or more FcRn binding loops derived from the Cγ2 and Cγ3 domains of IgG antibodies. The loops that are replaced in the Cg3 and Cε4 domains of IgE show structural homology to the FcRn binding loops in the Cγ2 and Cγ3 domains of IgG.
[0143] For comparison, two IgE fusion constructs were created in which i) the IgG-derived hinge and Cγ2 domains were fused to the C-terminus of trastuzumab IgE, and ii) the IgG hinge and Cγ2 and Cγ3 domains were fus...
Embodiment 2-I
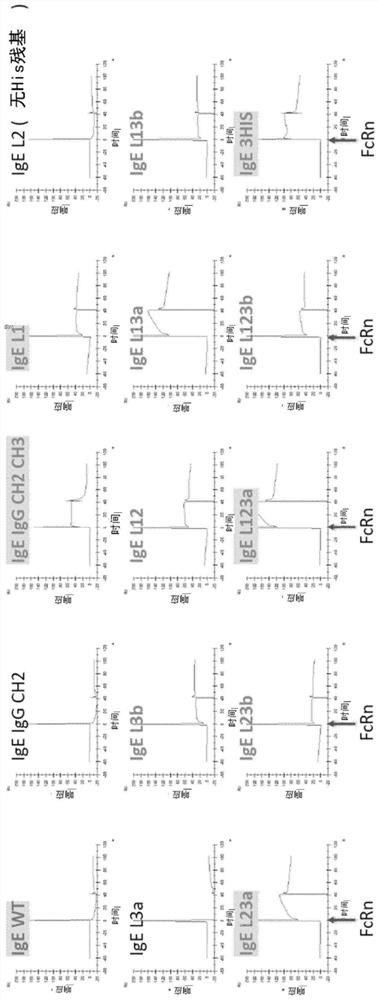
[0231] Example 2 - Binding of IgE variants to FcRn
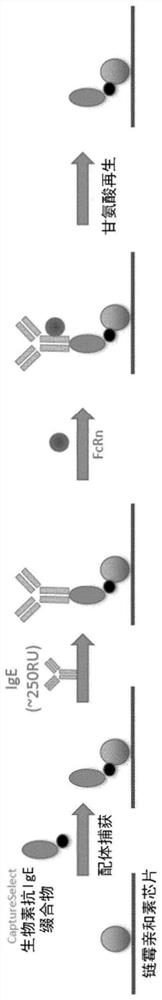
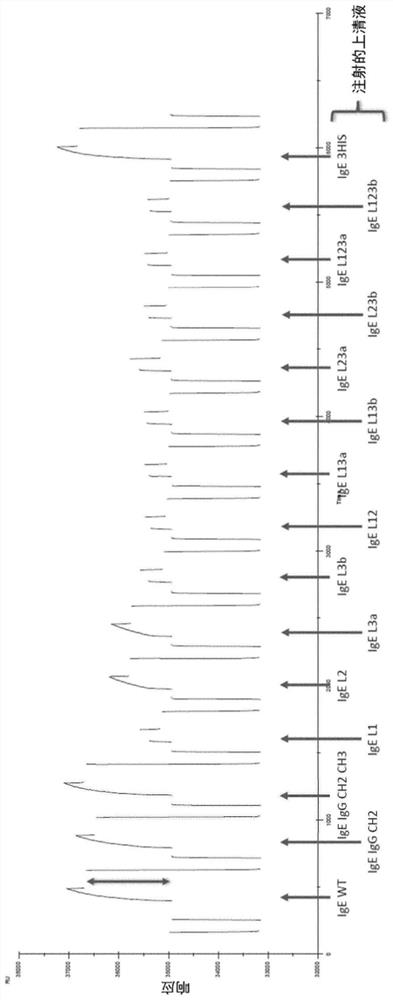
[0232] To assess the binding of antibody variants to FcRn (Sino Biological Cat. No. CT009-H08H), single concentration Biacore kinetic analysis was performed on supernatants from transfected CHO cell cultures. Kinetic experiments were performed on a Biacore T200 (serial number 1909913) running Biacore T200 Control software V2.0.1 and Evaluation software V3.0 (GE Healthcare, Uppsala, Sweden). The measurement principle is as figure 1 shown. All kinetic experiments were performed at 25°C using PBS containing 0.05% P20 (GE Healthcare, Little Chalfont, UK) and an additional 150 mM NaCl (pH 6.0). Antibodies were loaded on F of a Straptavidin chip (GE Healthcare, Little Chalfont, UK) preloaded with CaptureSelect biotin anti-IgE (Thermo cat no. 7103542500). c 2. F c 3 and F c 4 on. Antibody was captured at a flow rate of 10 μl / min to give a fixed level (RL) of ~250RU. Binding data were acquired using FcRn at 2000 nM at a flo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com