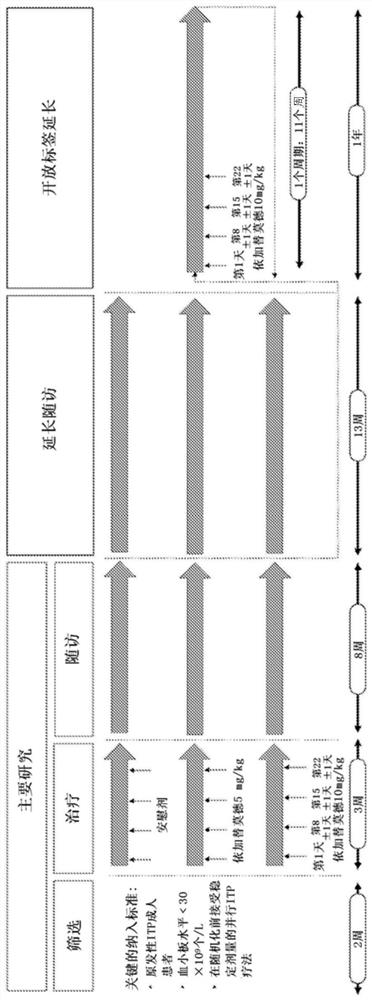
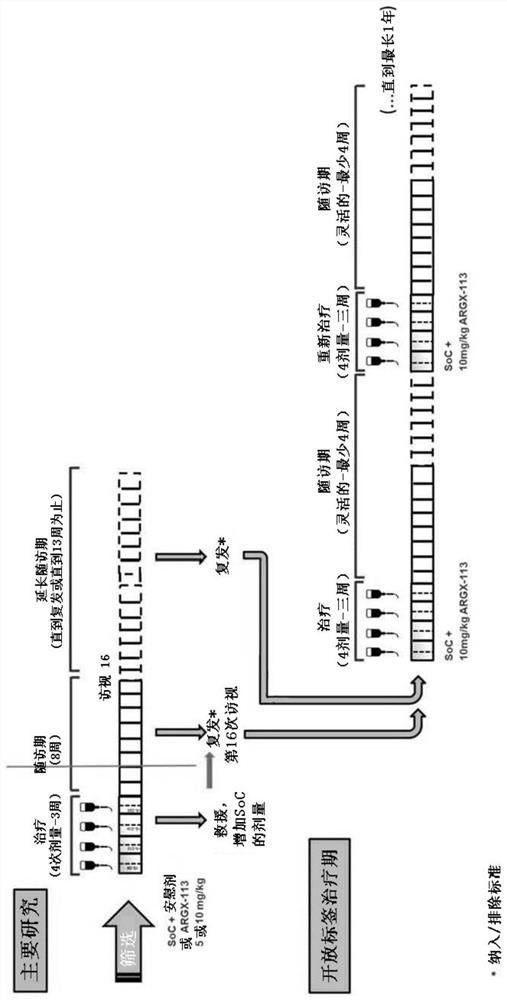
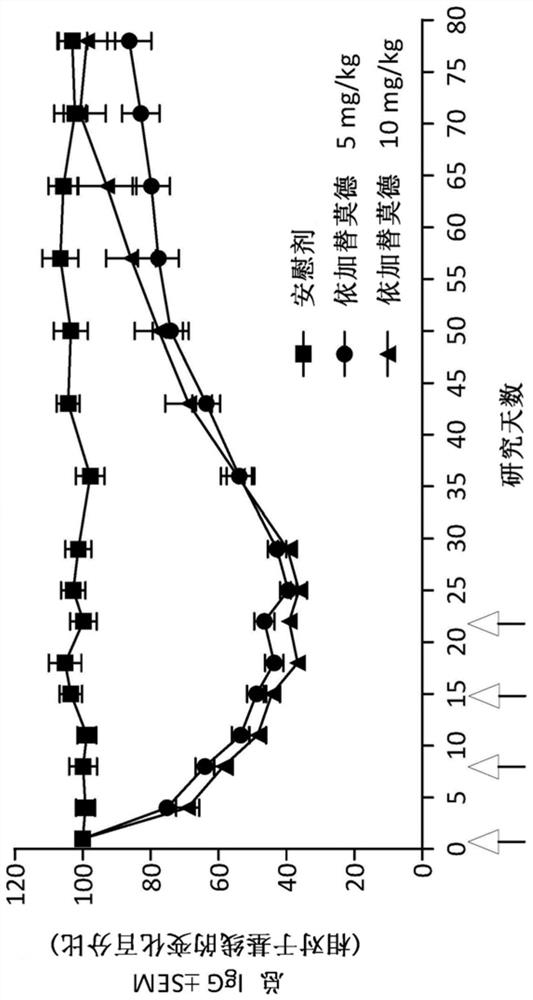
Compositions and methods for treating immune thrombocytopenia
A technology for thrombocytopenia, immunology, applied in the therapeutic field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153] Example 1: Single Dose Toxicity Study of ARGX-113 in Cynomolgus Monkeys
[0154] Four dose levels (10 mg, 30 mg, 50 mg, and 100 mg per kg body weight [b.w.]) of ARGX-113 and controls were administered to cynomolgus monkeys by 2-hour intravenous infusion. No signs of local intolerance associated with ARGX-113 were observed at any dose level tested. No associations with ARGX- 113 related effects. Furthermore, no macroscopic or microscopic systemic organ changes were observed in any of the animals examined, and in particular no histopathological changes were observed in the liver of monkeys at any dose level tested.
[0155] Administration of ARGX-113 caused significant changes in biochemical parameters that were not considered adverse in nature. A decrease in the serum concentration of γ-globulin was observed in all ARGX-113 treated groups. Since ARGX-113 increases antibody clearance by binding to the FcRn receptor, the decrease in total immunoglobulins (gamma-globuli...
Embodiment 2
[0157] Example 2: Repeated Dose Toxicity Study of ARGX-113 in Cynomolgus Monkeys
[0158] In a 1-month repeat-dose toxicology study in cynomolgus monkeys, ARGX-113 was administered at 3 dose levels (3 mg / kg, 30 mg / kg, and 100 mg / kg). Ten animals, 5 male and 5 female cynomolgus monkeys, were treated at each dose level and received intravenous infusions of ARGX-113 every 48 hours for a total of 15 infusions. ARGX-113 was well tolerated by all animals at all doses as determined by clinical signs, body weight, macroscopic examination, histopathology, food consumption, and hematological and serum biochemical parameters. No macroscopic changes associated with ARGX-113 were observed. Histopathological examination revealed changes in the liver at a dose of 100 mg / kg ARGX-113. Liver changes included cytoplasmic changes and degeneration and a diffuse mixed inflammatory cell infiltration. There were no liver lesions in animals in the 100 mg / kg dose group at the end of the treatment-fr...
Embodiment 3
[0167] Example 3: Repeated Dose Toxicity Study of ARGX-113 in Rats
[0168] In a 1-month repeated-dose toxicology study in rats, ARGX-113 was administered at 3 dose levels (10 mg / kg, 30 mg / kg, and 100 mg / kg). Twenty animals, ten males and ten females, were treated at each dose level and received intravenous injections of ARGX-113 every 48 hours for a total of 15 infusions. ARGX-113 was well tolerated by all animals at all doses as determined by clinical signs, body weight, macroscopic examination, histopathology, food consumption, and hematological and serum biochemical parameters. No macroscopic changes associated with ARGX-113 were observed. Histopathological examination revealed test article-related histopathological lesions in the liver at doses of 100 mg / kg ARGX-113 in some animals. The lesion consisted of Kupffer cell hypertrophy / hyperplasia, which was observed in both females and males in the 100 mg / kg ARGX-113 treated group. There were no liver lesions in animals in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com