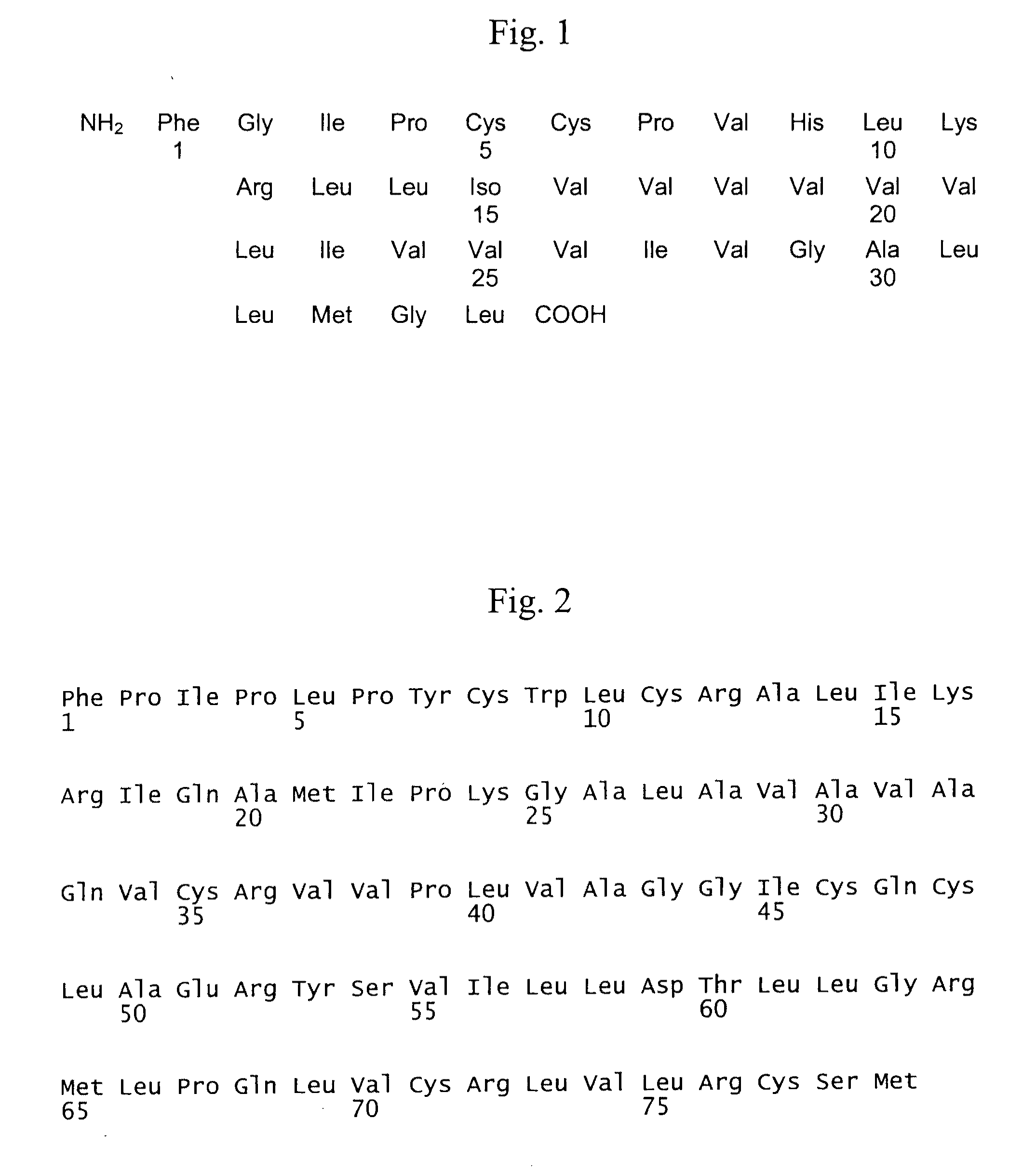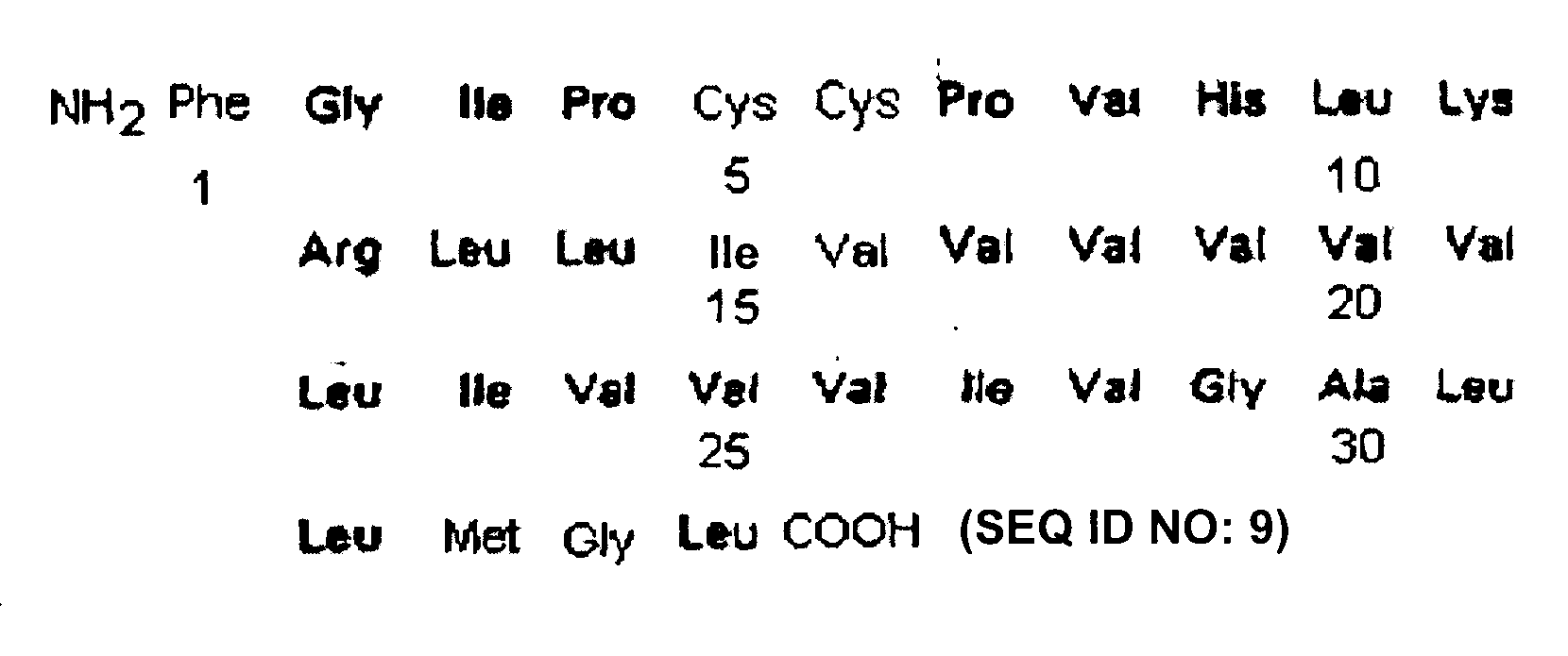Patents
Literature
114results about "Alveolar/pulmonary surfactant peptides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
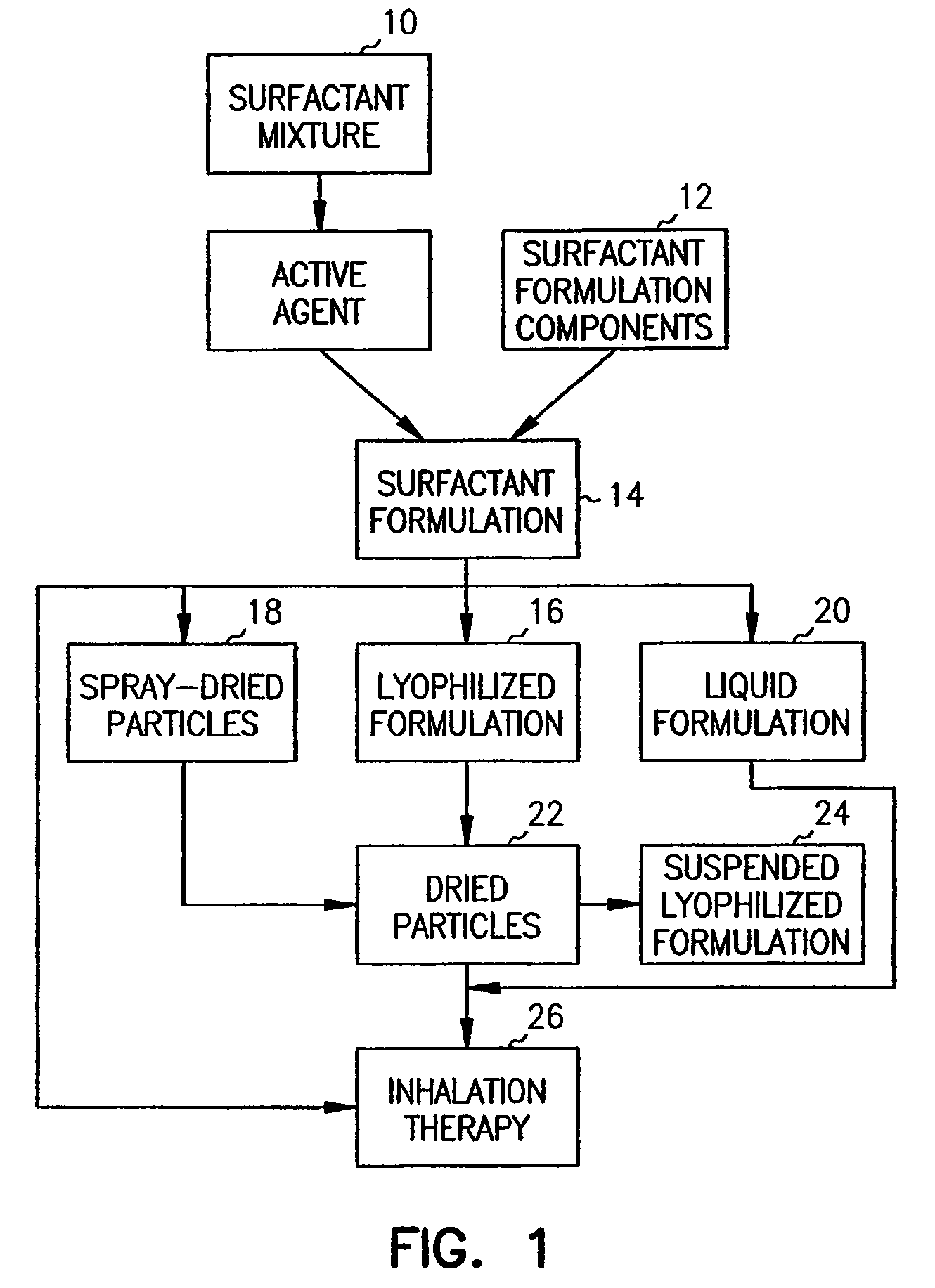
Methods, systems and devices for noninvasive pulmonary delivery
InactiveUS20060078506A1Sufficient amountFacilitate communicationRespiratorsPowder deliveryActive agentAnesthesia
Owner:DISCOVERY LABORATORIES INC
Reconstituted surfactants having improved properties
ActiveUS7897577B2Peptide/protein ingredientsAlveolar/pulmonary surfactant peptidesSURFACTANT BLENDRepeat unit
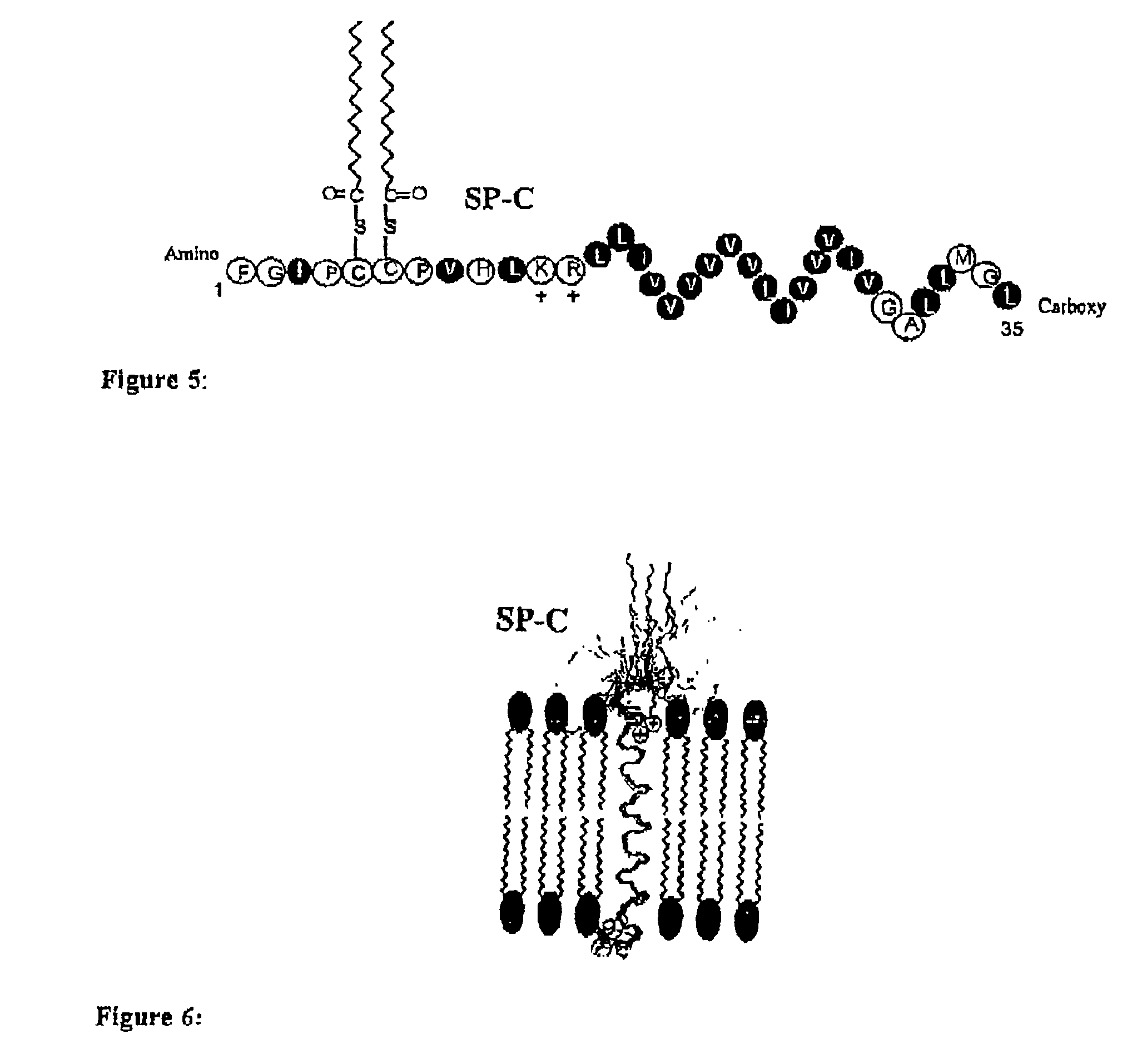
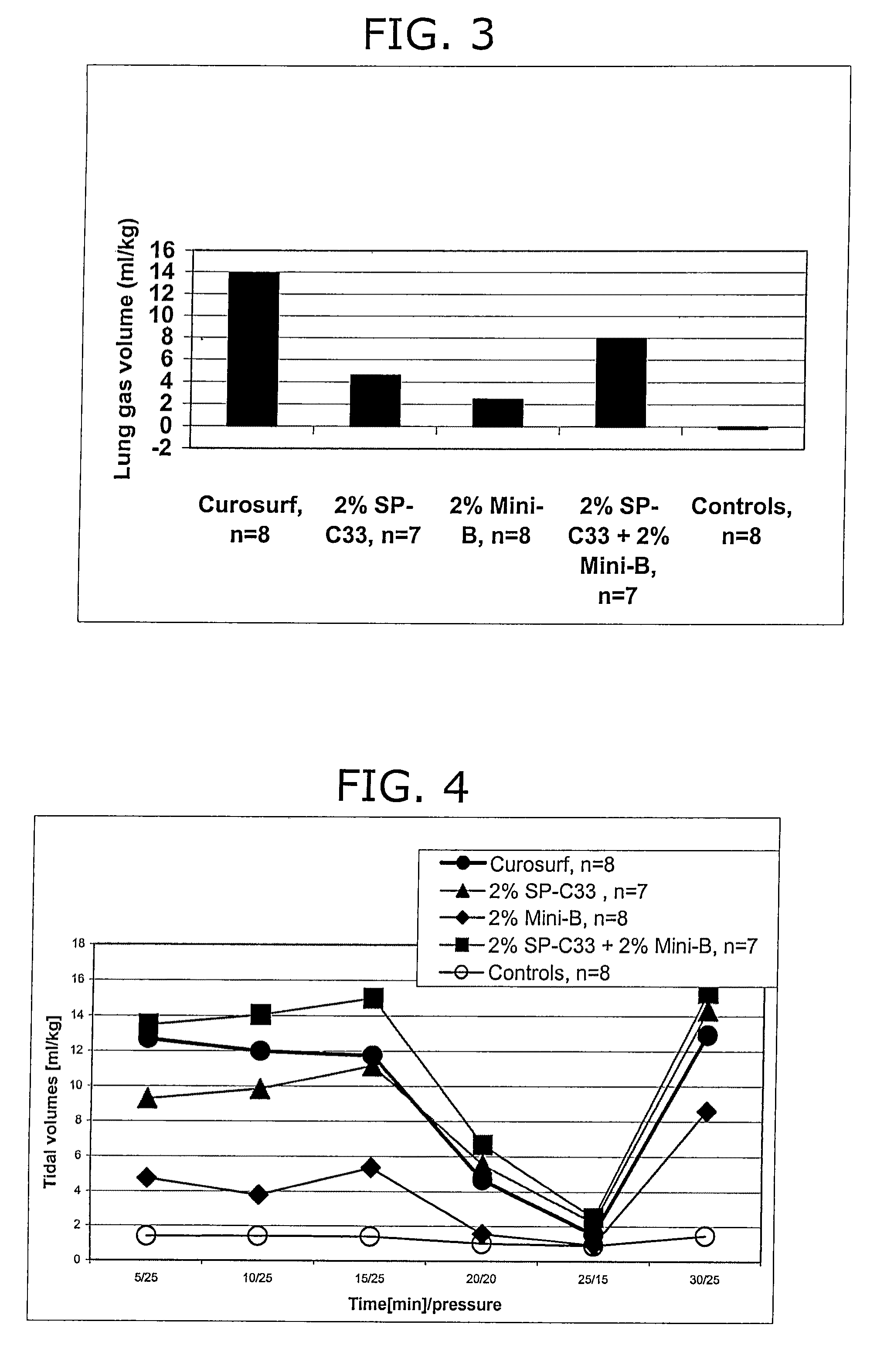
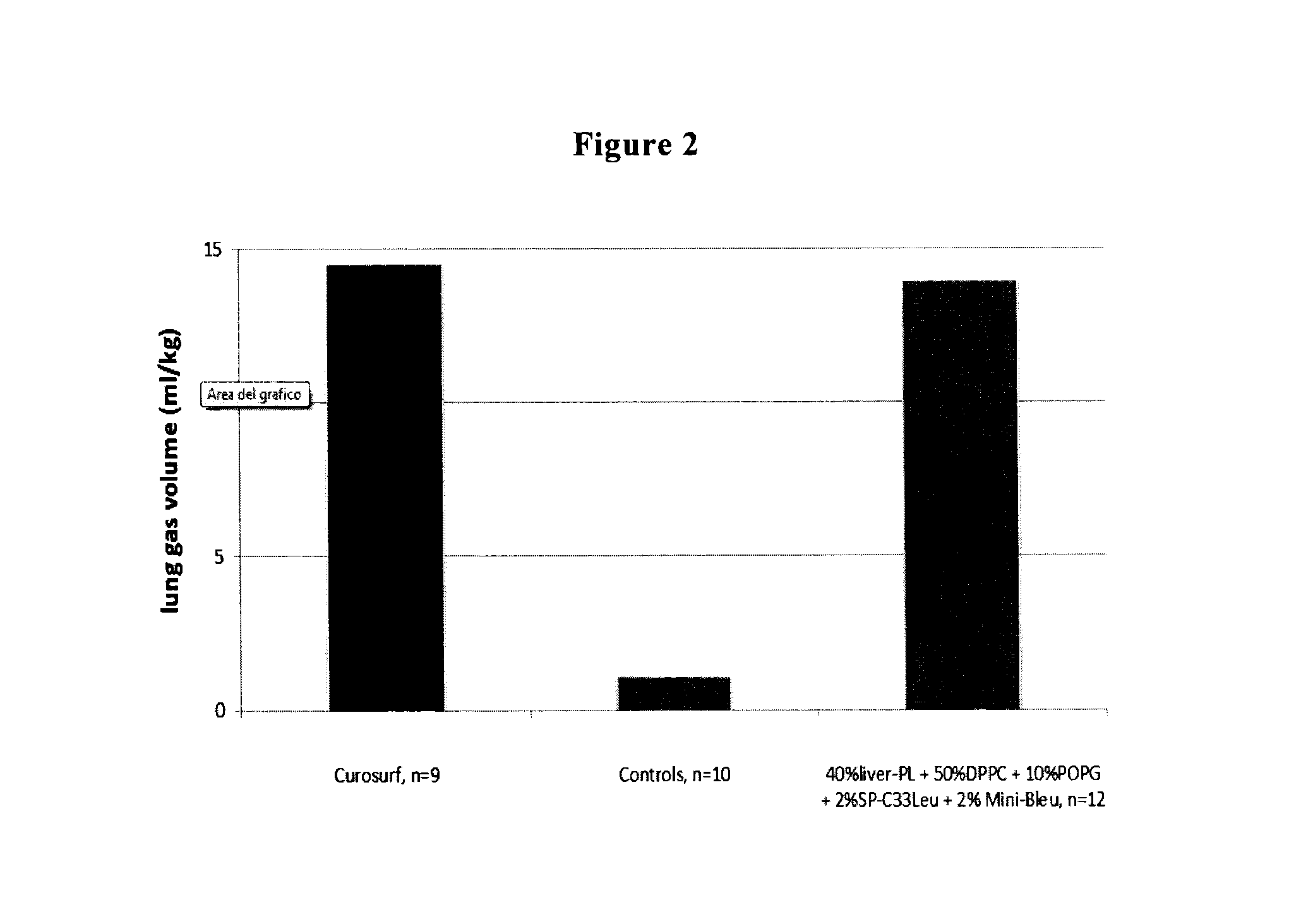
The present invention is directed to a reconstituted surfactant containing a lipid carrier, a polypeptide analog of the native surfactant protein SP-C, and a polypeptide comprising a sequence comprised of repeated units where each unit contains between 3 and 8 hydrophobic amino acid residues and one basic amino acid residue. The invention is also directed to the pharmaceutical compositions thereof and to its use for the prophylaxis and / or treatment of RDS and other respiratory disorders.
Owner:CHIESI FARM SPA
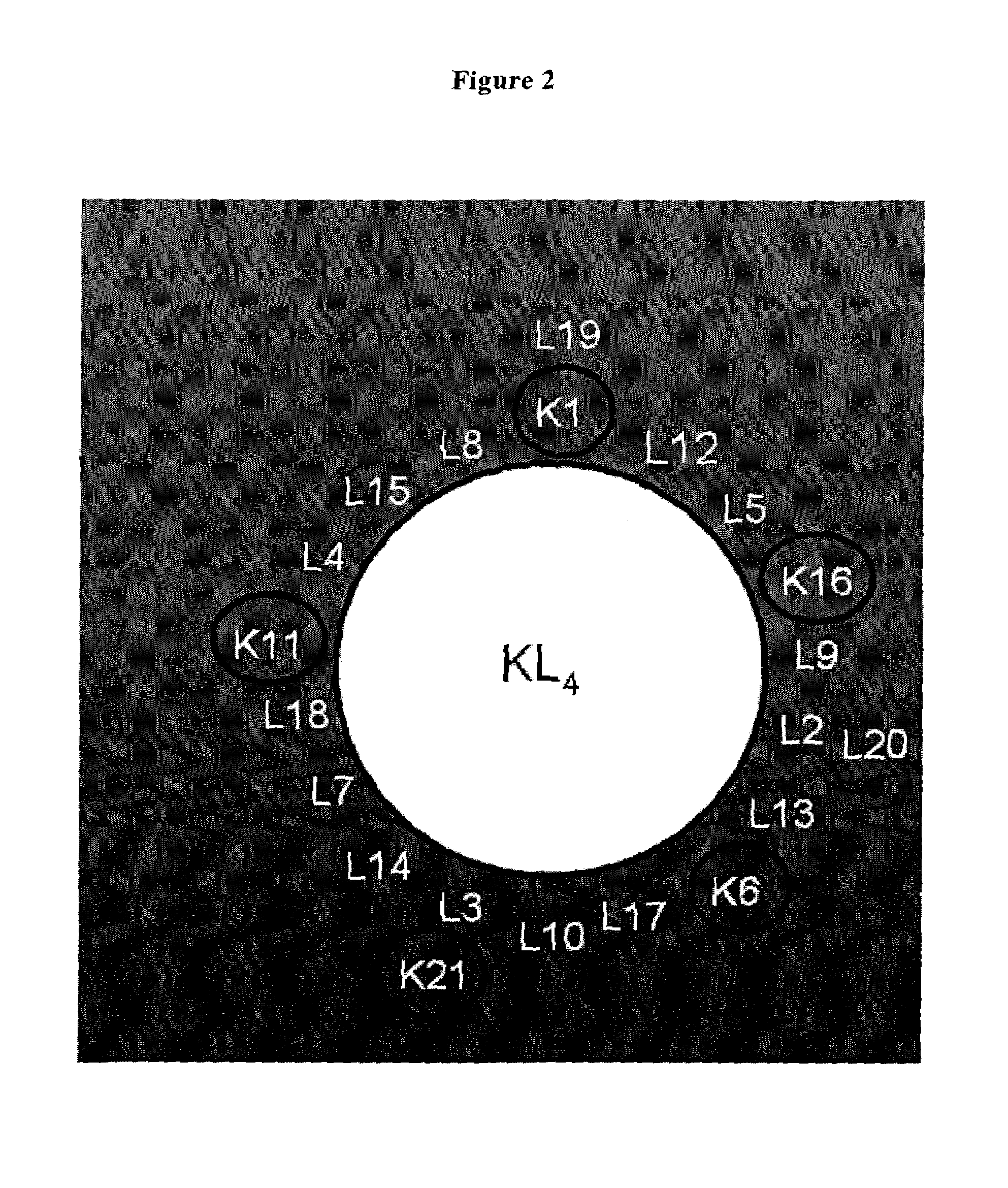
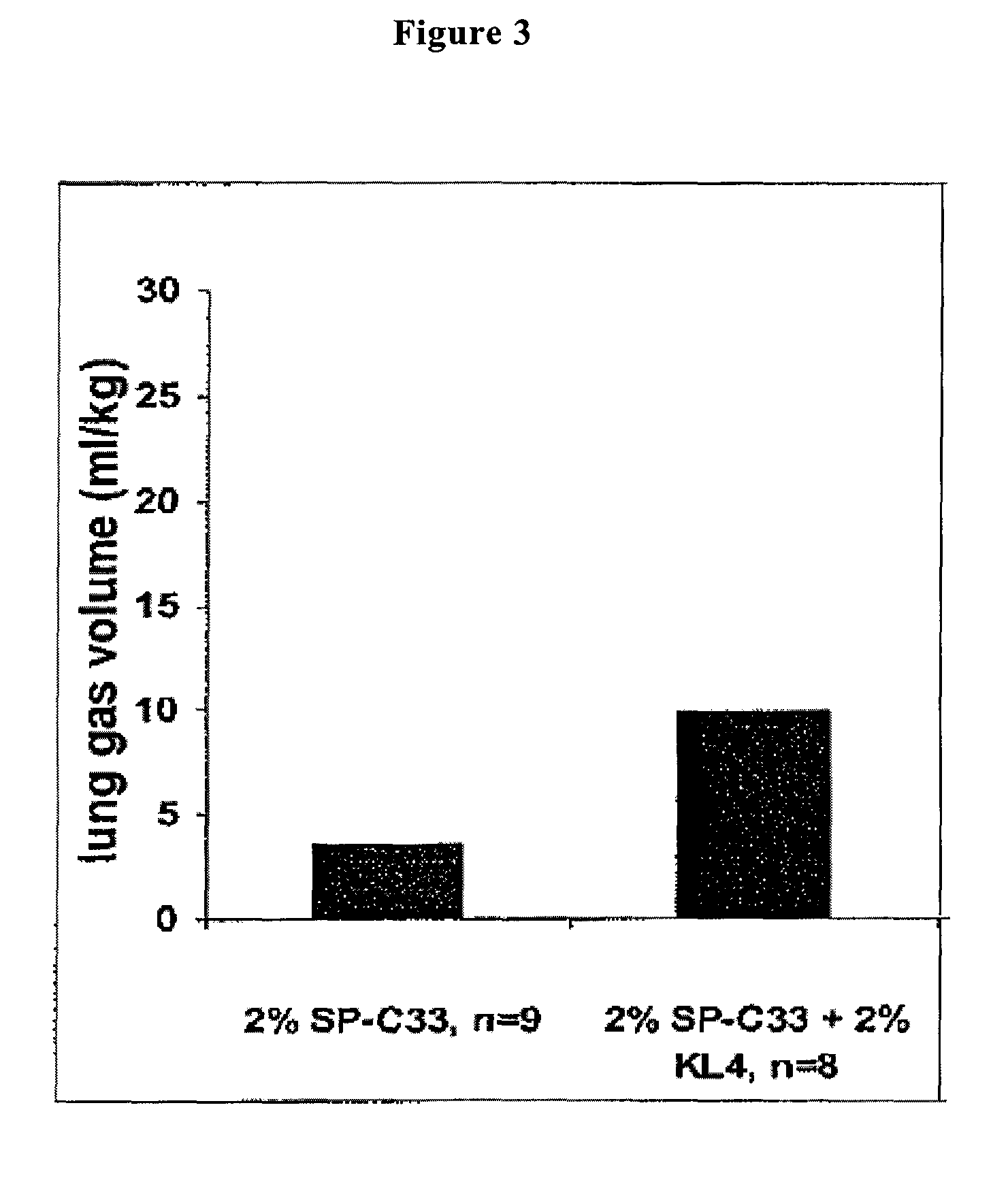
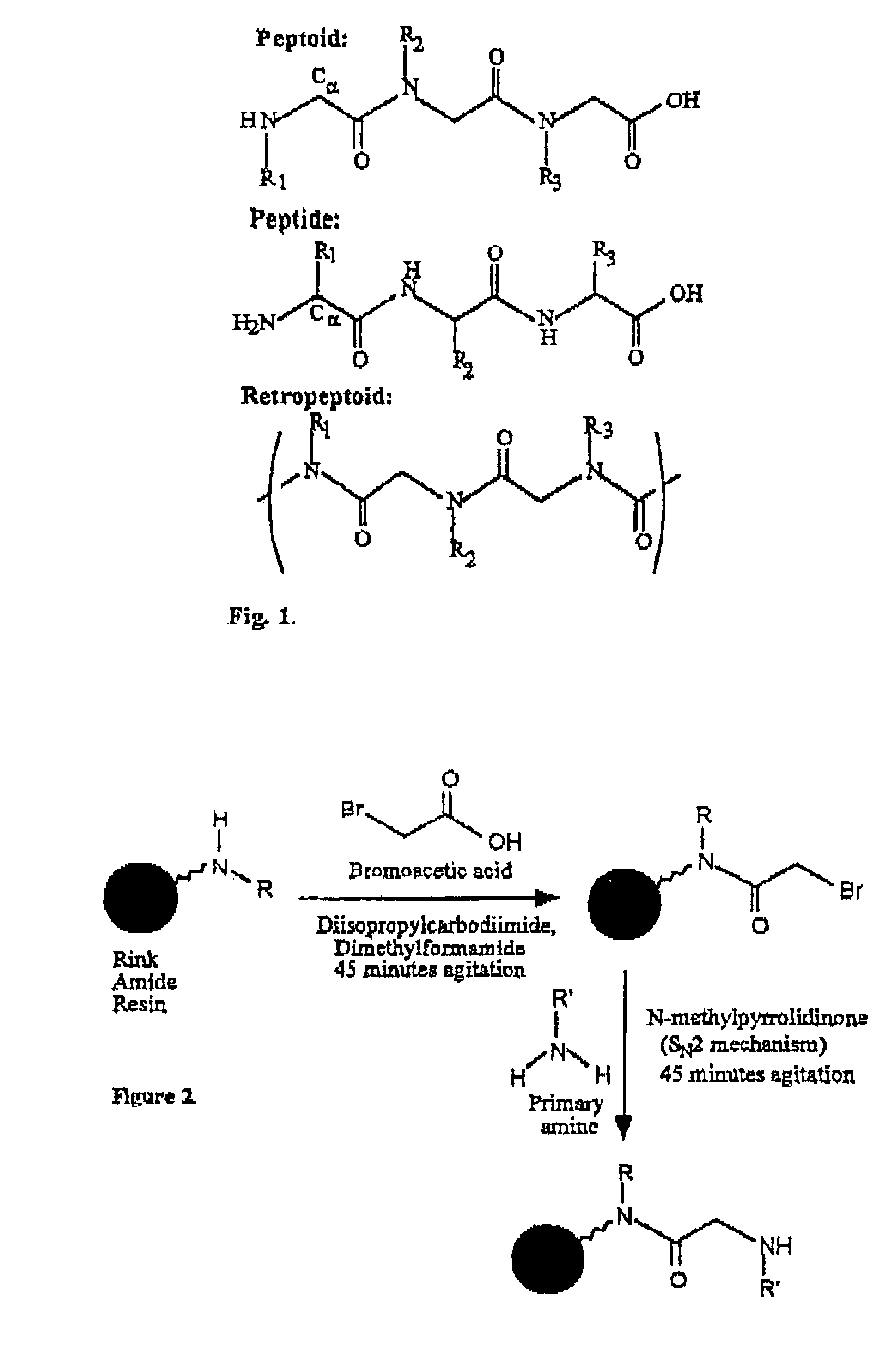
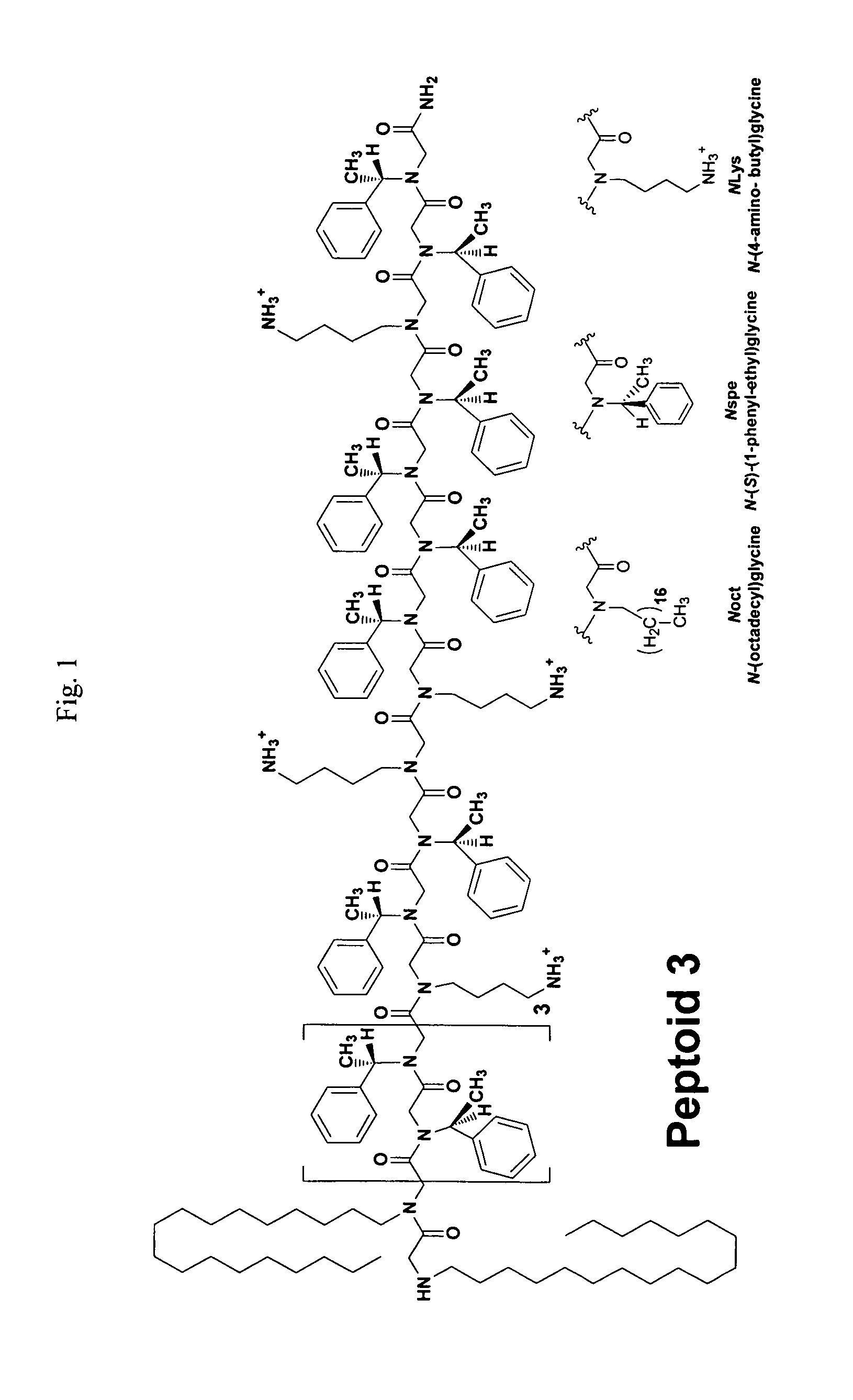
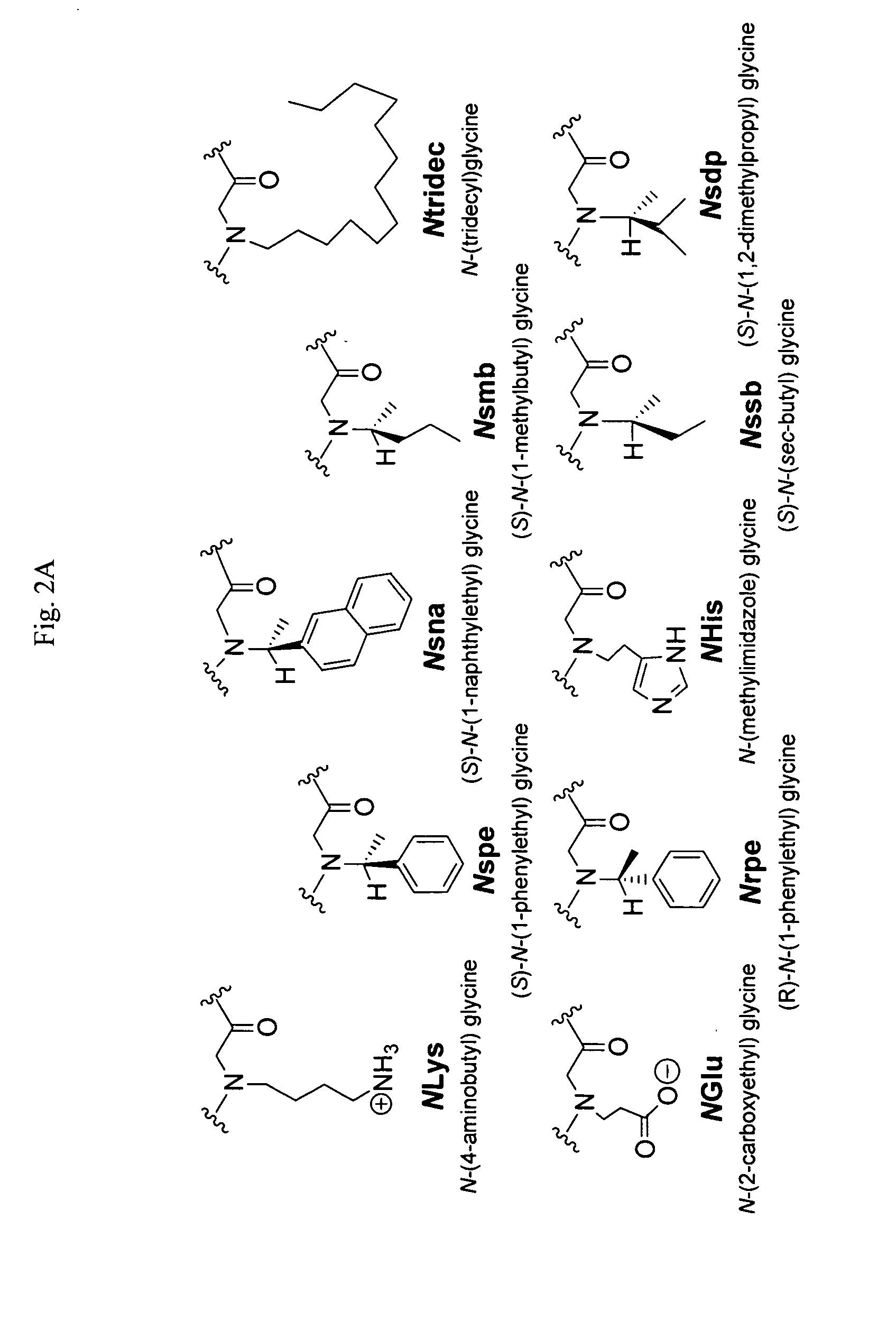
Polypeptoid pulmonary surfactants
InactiveUS6887845B2Low immunogenicityImprove bioavailabilityBiocidePeptide/protein ingredientsOligomerReverse transcriptase
The present invention provides spreading agents based on sequence-specific oligomers comprising a peptoid, a peptide-peptoid chimera, a retropeptoid or a retro(peptoid-peptide) chimera, and methods for using the same, including for the treatment of respiratory distress of the lungs. The spreading agents are sequence-specific oligomers, including retrosequence-specific oligomers, based on a peptide backbone, that are designed as analogs of surfactant protein-B or surfactant protein-C.
Owner:BARRON PH D ANNELISE E +1
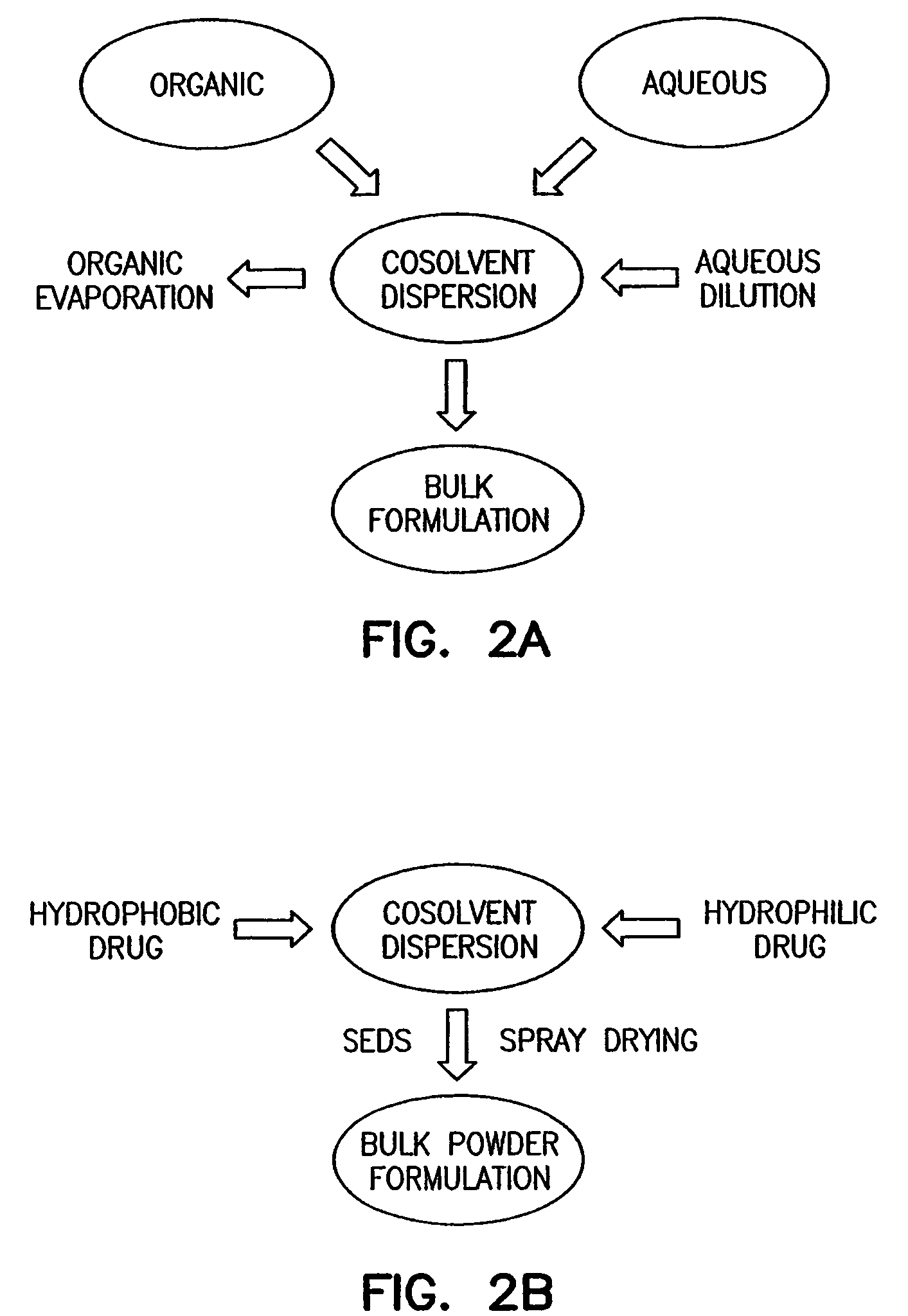
Process for the production of powdered pulmonary surfactant preparations
A powdered pulmonary surfactant preparation containing a hydrophobic protein serving as a pulmonary surfactant is obtained by spray drying an organic solution or suspension containing a hydrophobic protein serving as a pulmonary surfactant and possibly other components. Obtained power preparations exhibit very good stability under storage, are easy to reconstitute and are also suitable for administration by inhalation.
Owner:TAKEDA GMBH
Pulmonary surfactant formulations
InactiveUS20060286038A1Increase ratingsImprove drug stabilityBiocideOrganic active ingredientsDiseaseDipalmitoyl Phosphatidylcholine
Synthetic pulmonary surfactant compositions comprising dipalmitoyl phosphatidylcholine, phosphatidylglycerol, and essentially neutral lipid, and having essentially no 1-palmitoyl 2-oleoyl phosphatidylglycerol and essentially no palmitic acid are provided. Methods for treating respiratory disease are also provided comprising administering a therapeutically effective amount of a synthetic pulmonary surfactant comprising dipalmitoyl phosphatidylcholine, phosphatidylglycerol, and essentially neutral lipid, and having essentially no 1-palmitoyl 2-oleoyl phosphatidylglycerol and essentially no palmitic acid.
Owner:DISCOVERY LABORATORIES INC
Compositions for the treatment of ARDS or IRDS containing 3-(cycloproplymethoxy)-N-(3,5-dichloro-4-pyridinyl)-4-(difluoromethoxy) benzamide and lung surfactant
Owner:ASTRAZENECA AB
Use of recombinant human uteroglobin in treatment of inflammatory and fibrotic conditions
InactiveUS20050261180A1Lower Level RequirementsReduce inflammationPeptide/protein ingredientsMicrobiological testing/measurementFibrosisIn vivo
Methods for treatment of inflammatory and fibrotic conditions in vivo using UG is disclosed. Methods for treating or preventing inflammatory or fibrotic conditions characterized by a deficiency of endogenous functional UG are also disclosed. Compositions containing UG, optionally containing lung surfactant, and assay procedures for detection of UG-fibronectin complexes, are also provided.
Owner:CC10 SWEDEN
Diagnosis, prognosis and treatment of pulmonary diseases
InactiveUS20060078558A1Reducing airway resistance responseImprove responsivenessOrganic active ingredientsPeptide/protein ingredientsDiseaseObstructive Pulmonary Diseases
The present invention provides methods to protect a subject from a respiratory disorder involving an airway obstructive disease such as asthma or chronic obstructive pulmonary disease. Provided are methods to protect a subject from an airway obstructive disease using gene therapy. Methods are provided for supplying FoxA2 function to cells of the lung and airway, such as smooth muscle and epithelial cells, by FoxA2 gene therapy. The FoxA2 gene, a modified FoxA2 gene, or a part of the gene may be introduced into the cell in a vector such that the gene remains extrachromosomal or may be integrated into the subjects chromosomal DNA for expression. These methods provide for administering to a subject in need of such treatment a therapeutically effective amount of a FoxA2 gene, or pharmaceutically acceptable composition thereof, for overexpressing the FoxA2 gene. Such methods of expressing the administered FoxA2 gene in the lungs and airway provide for: (1) preventing or alleving bronchial hyperresponsiveness; (2) preventing or alleving of an airway obstructive disease, e.g., bronchial hyperreactivity, airway hyperresponsiveness, asthma or chronic obstructive pulmonary disorder (“COPD”); (3) reducing the airway resistance response to inhaled natural or synthetic bronchoconstrictors or allergens or to exercise; and (4) enhancing responsiveness (relaxation) of airway tissues to β-agonists.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Composition comprising a pulmonary surfactant and a pde5 inhibitor for the treatment of lung diseases
InactiveUS20060148693A1Preventing and reducing onsetReduce severityBiocidePeptide/protein ingredientsPhosphodiesterase 5 inhibitorPde5 inhibition
The invention relates to the combined administration of a pulmonary surfactant and a PDE5 inhibitor for the treatment of a disease in which pulmonary surfactant malfunction and / or phosphodiesterase 5 (PDE5) activity is detrimental.
Owner:TAKEDA GMBH
Surfactant protein-d for prevention and treatment of lung infections and sepsis
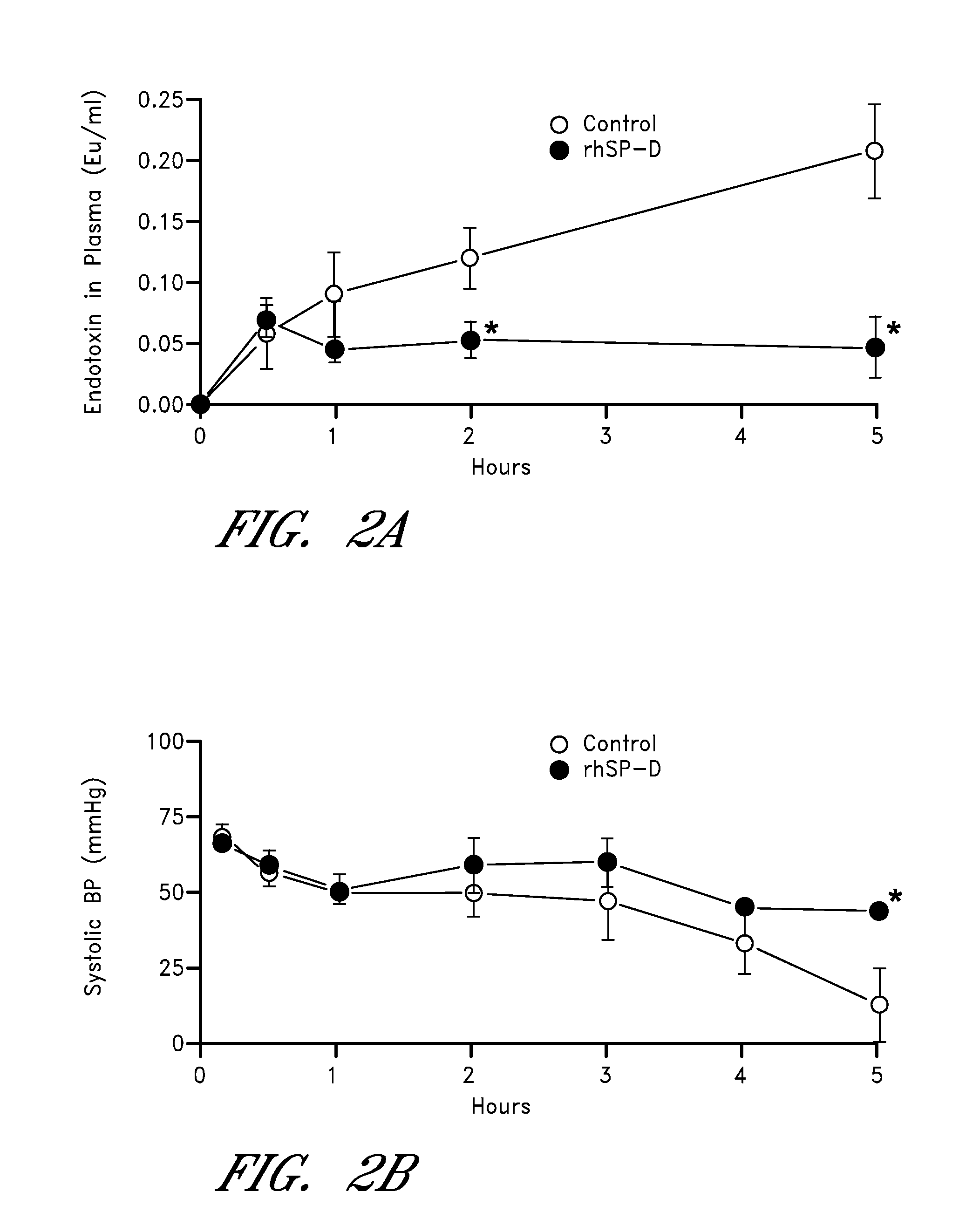
InactiveUS20080242615A1Reduce leakageDecrease endotoxin levelAntibacterial agentsPeptide/protein ingredientsCollectinADAMTS Proteins
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
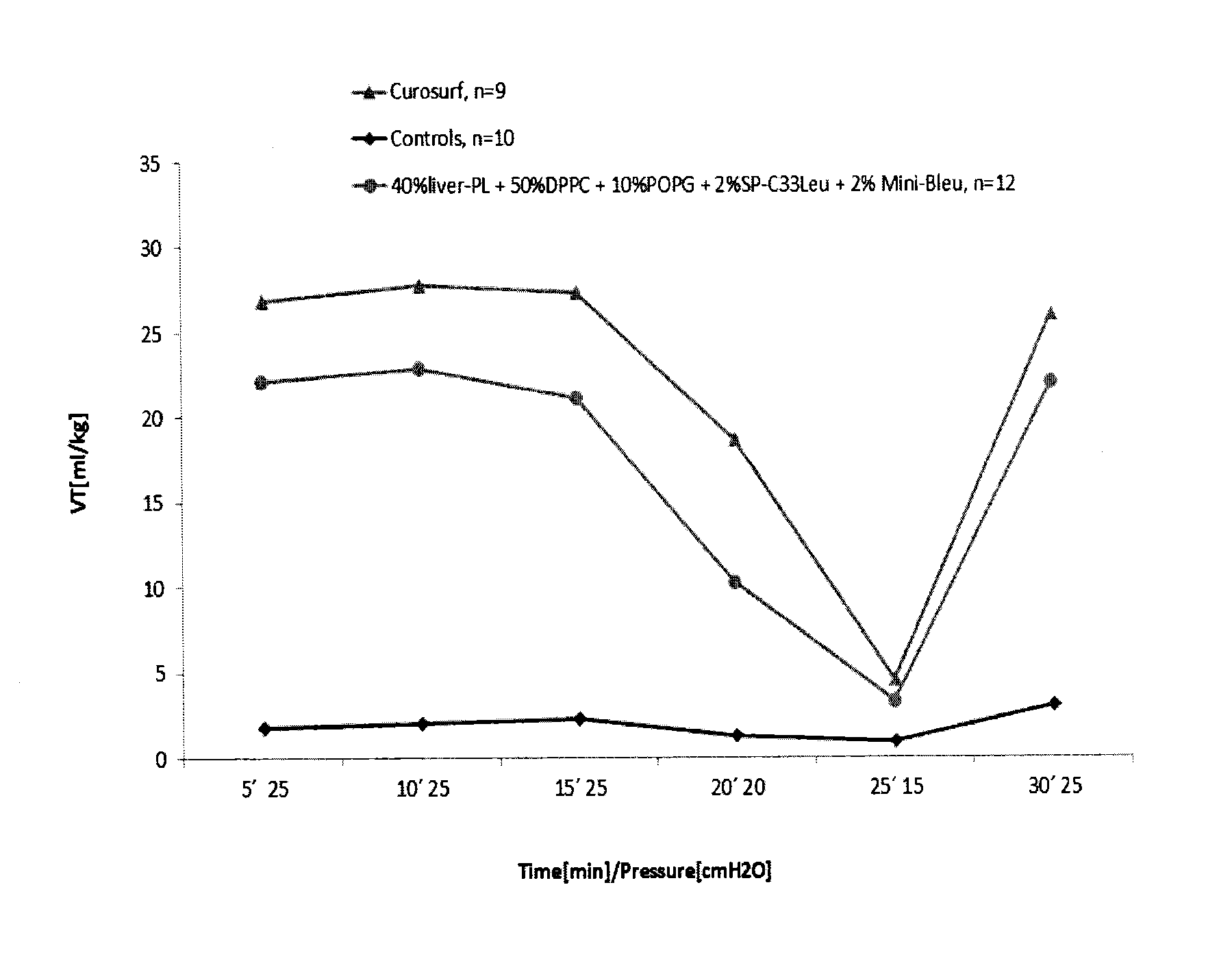
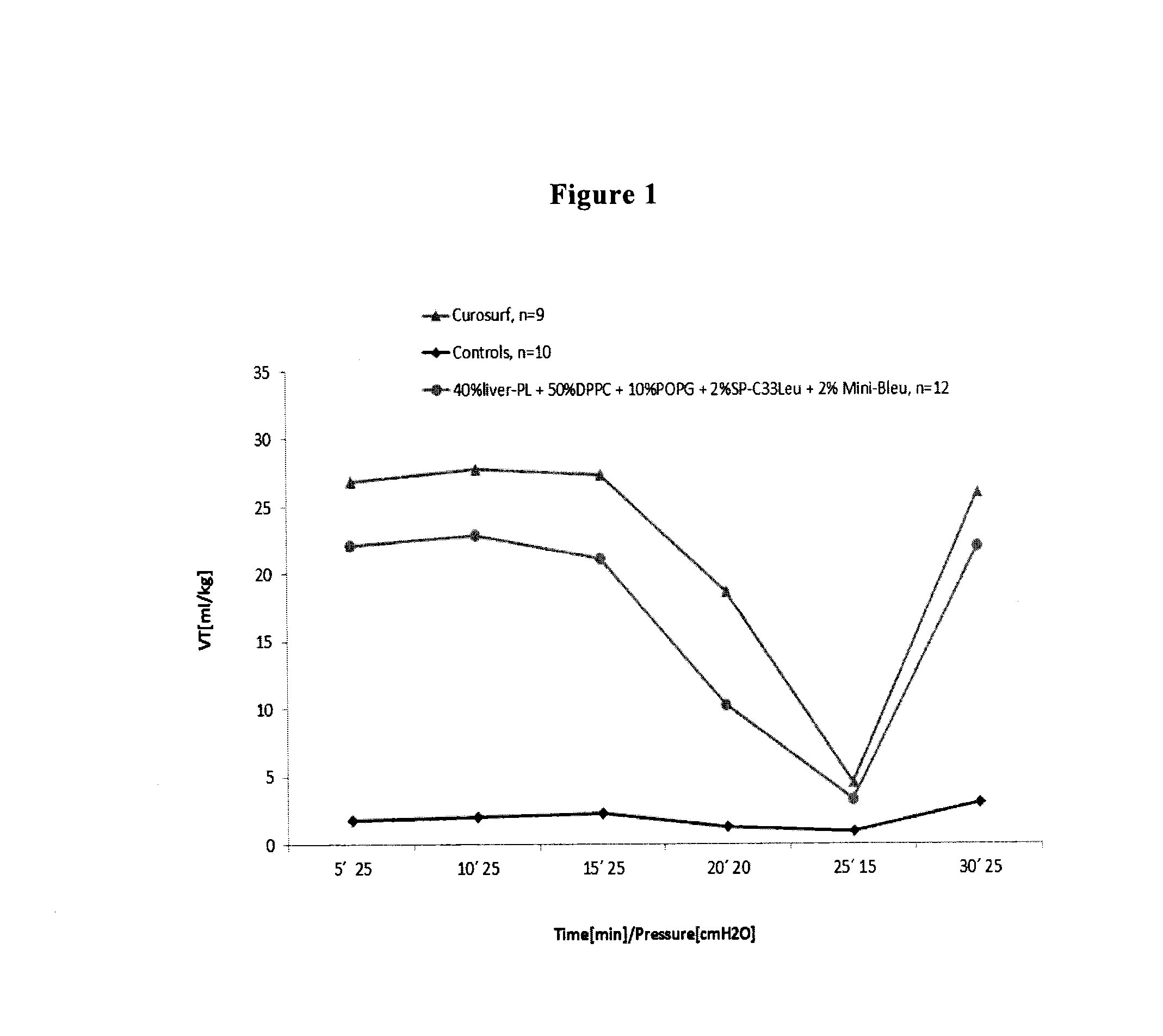
Synthetic lung surfactant and use thereof
ActiveUS20100055164A1Improve surface activityReduce surface tensionBiocidePeptide/protein ingredientsGlycerol DerivativesPhospholipase
The present invention relates to synthetic lung surfactant compositions that contain one or more of phospholipase-resistant phospho-glycerol derivatives, phospholipase-resistant phospho-choline derivatives, and surface active proteins or peptides, more preferably a combination of at least two or all three of these materials. Novel phospholipase-resistant phospho-glycerol derivatives, phospholipase-resistant phospho-choline derivatives, and surface active peptides are also disclosed herein. Uses of the surfactant compositions of the present invention to treat endogenous surfactant dysfunctional or deficient lung tissue, to prepare synthetic peptides for use in the surfactant compositions, and to deliver therapeutic agents are also disclosed.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT +2
Synthetic lipid mixtures for the preparation of a reconstituted surfactant
ActiveUS20050176625A1Organic active ingredientsBiocideLipid formationNaturally occurring surfactants
The invention relates to reconstituted surfactants consisting of artificial phospholipids and peptides able to lower the air-liquid surface tension, more particularly to reconstituted surfactants, comprising special phospholipid mixtures and artificial peptides which are analogues of the natural surfactant SP-C protein for the treatment of respiratory distress syndrome (RDS) and other diseases relates to pulmonary surfactant dysfunctions.
Owner:CHIESI FARM SPA
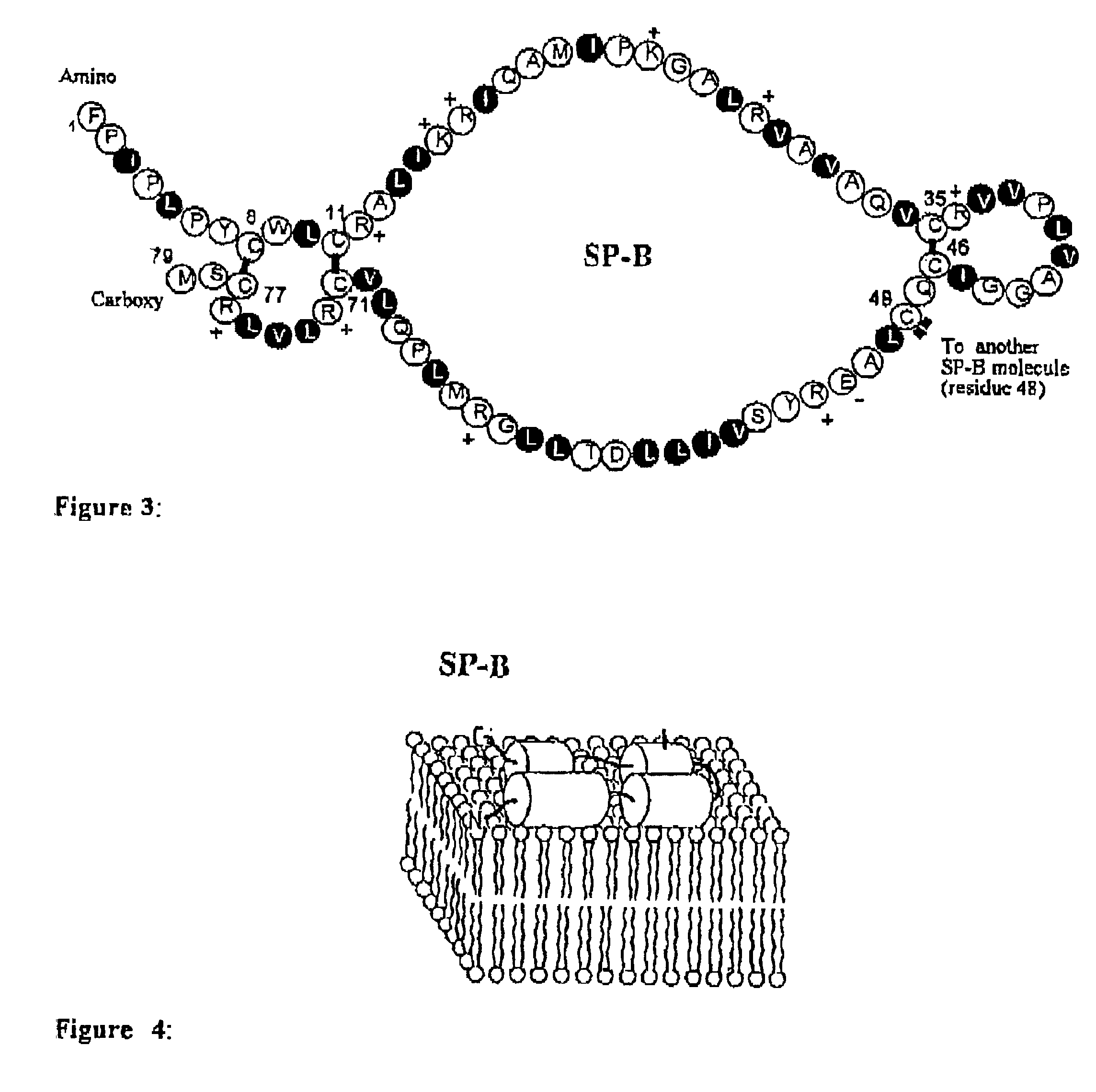
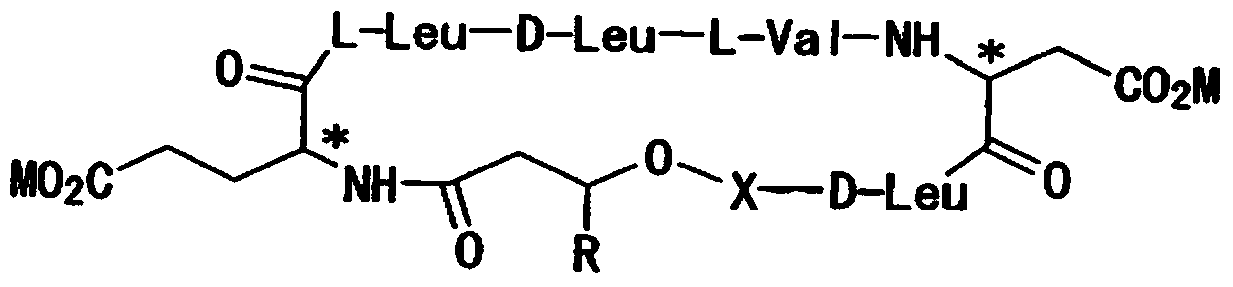
Exogenous surfactant protein B mimic
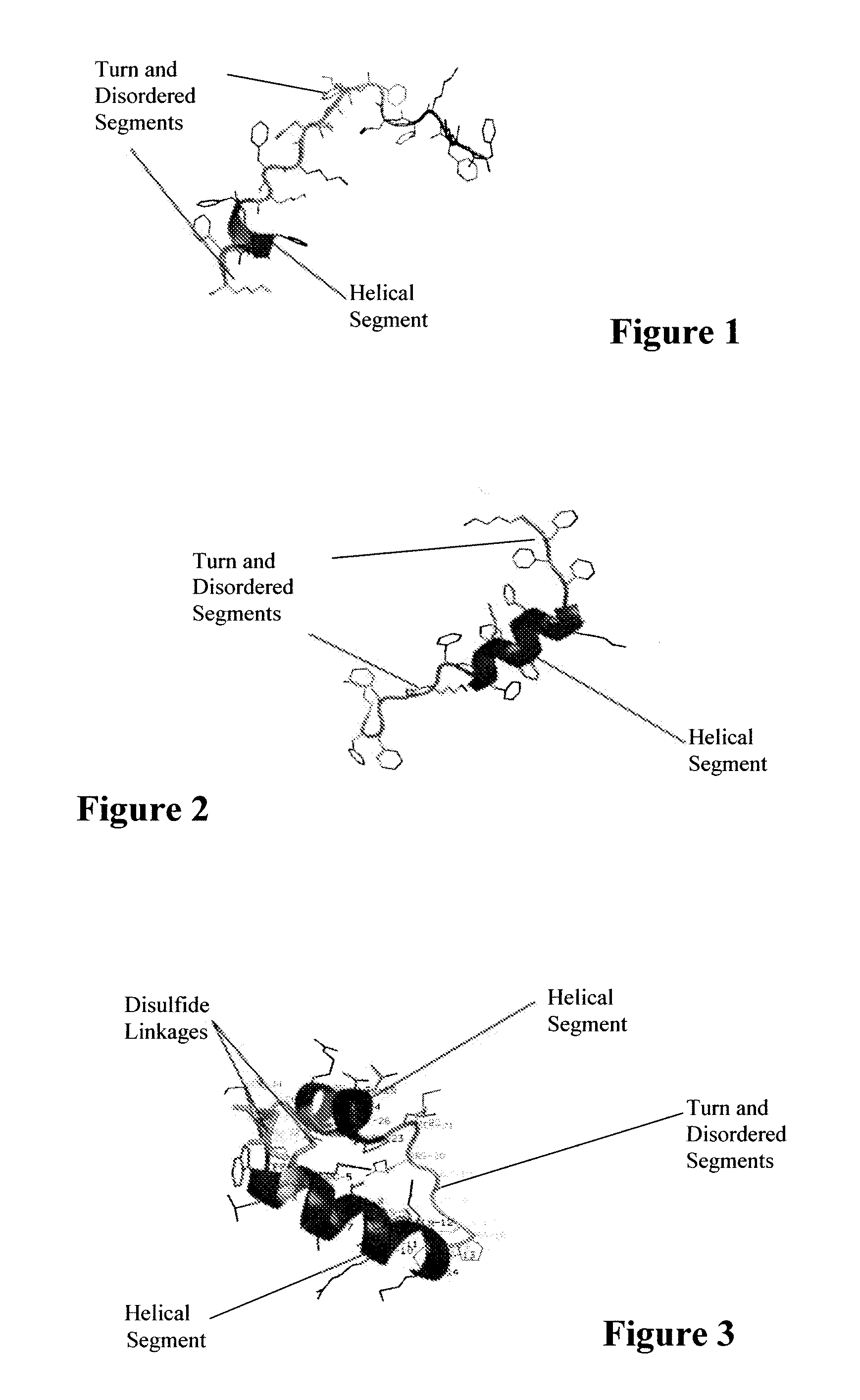
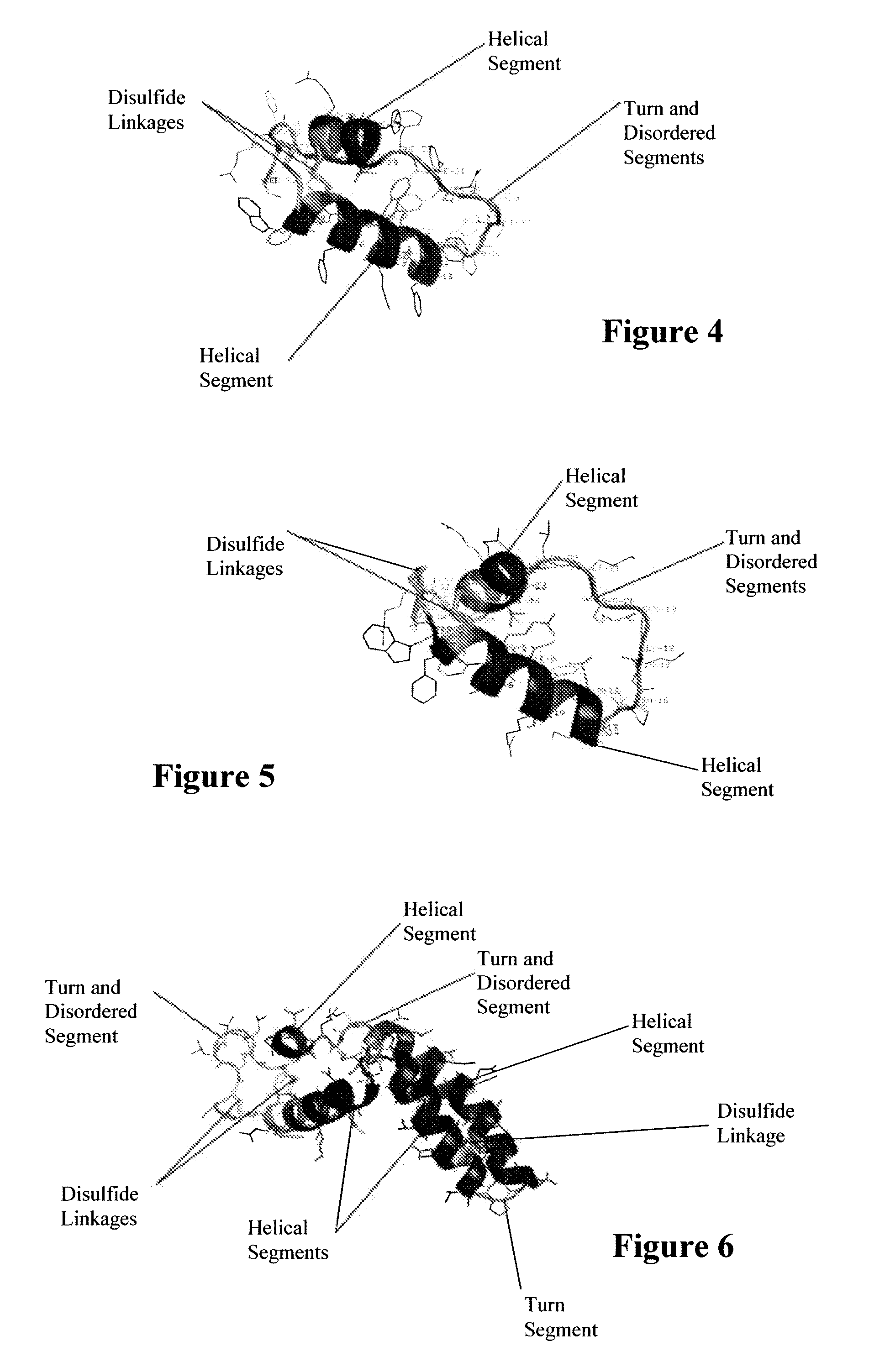
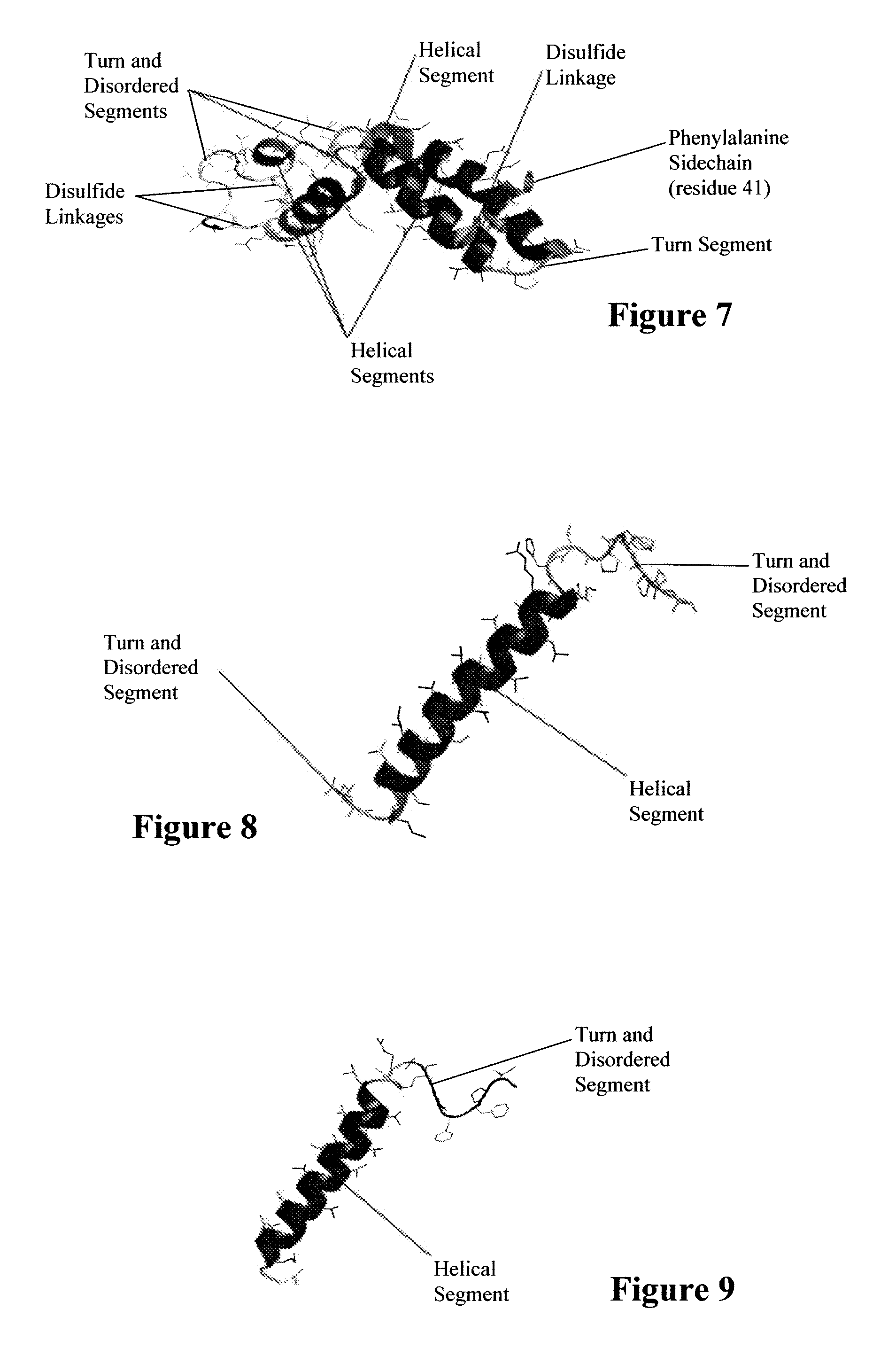
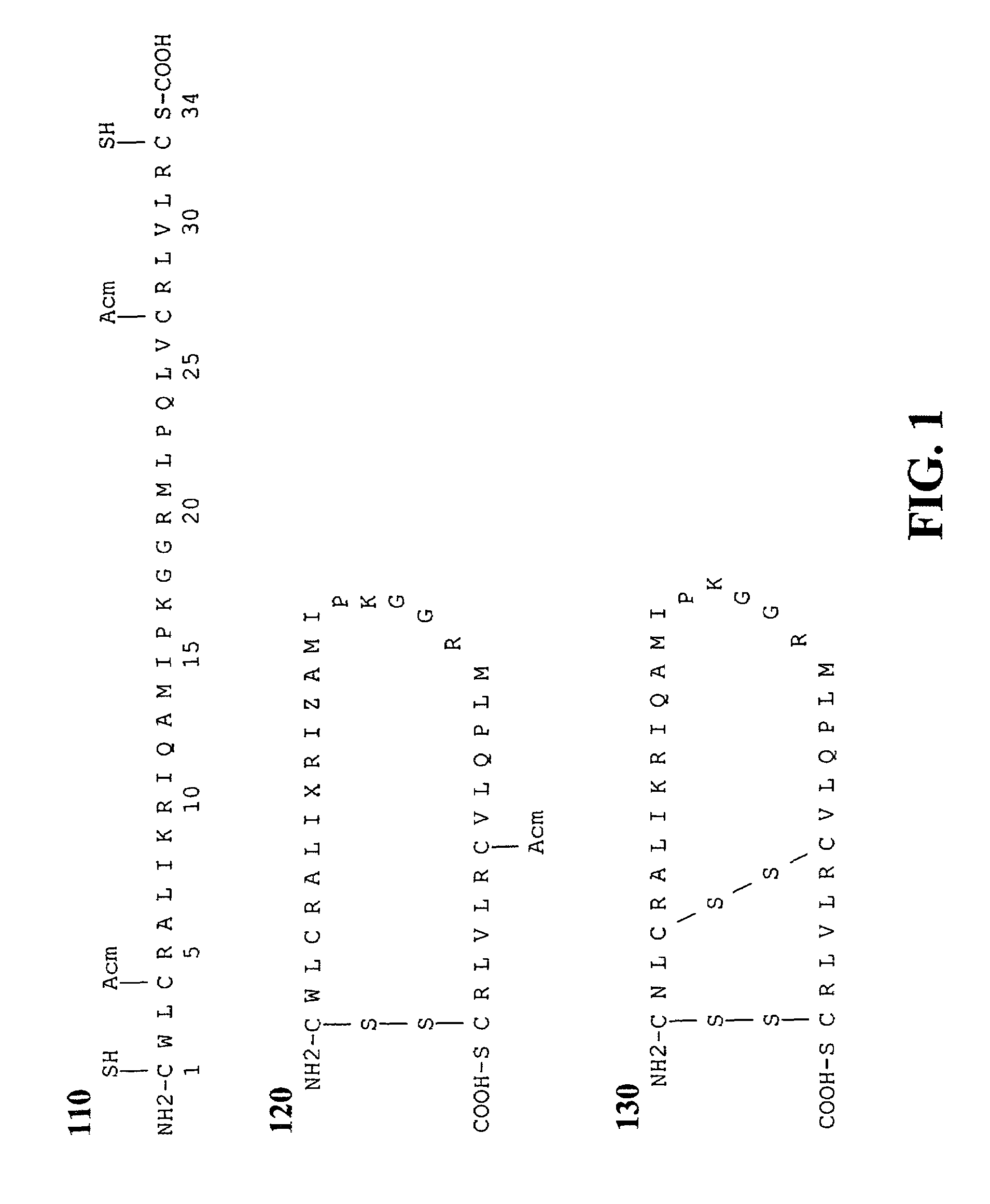
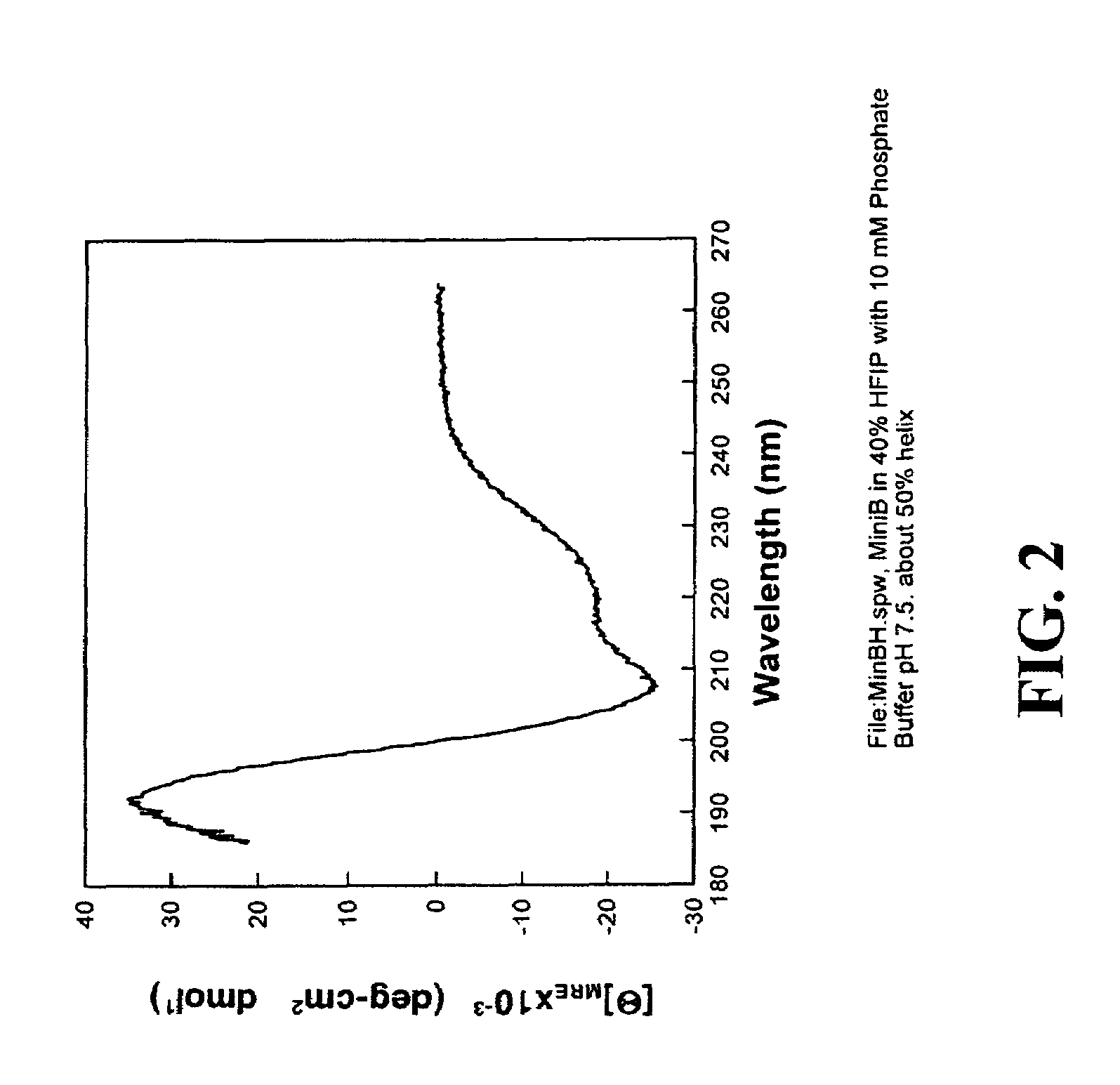
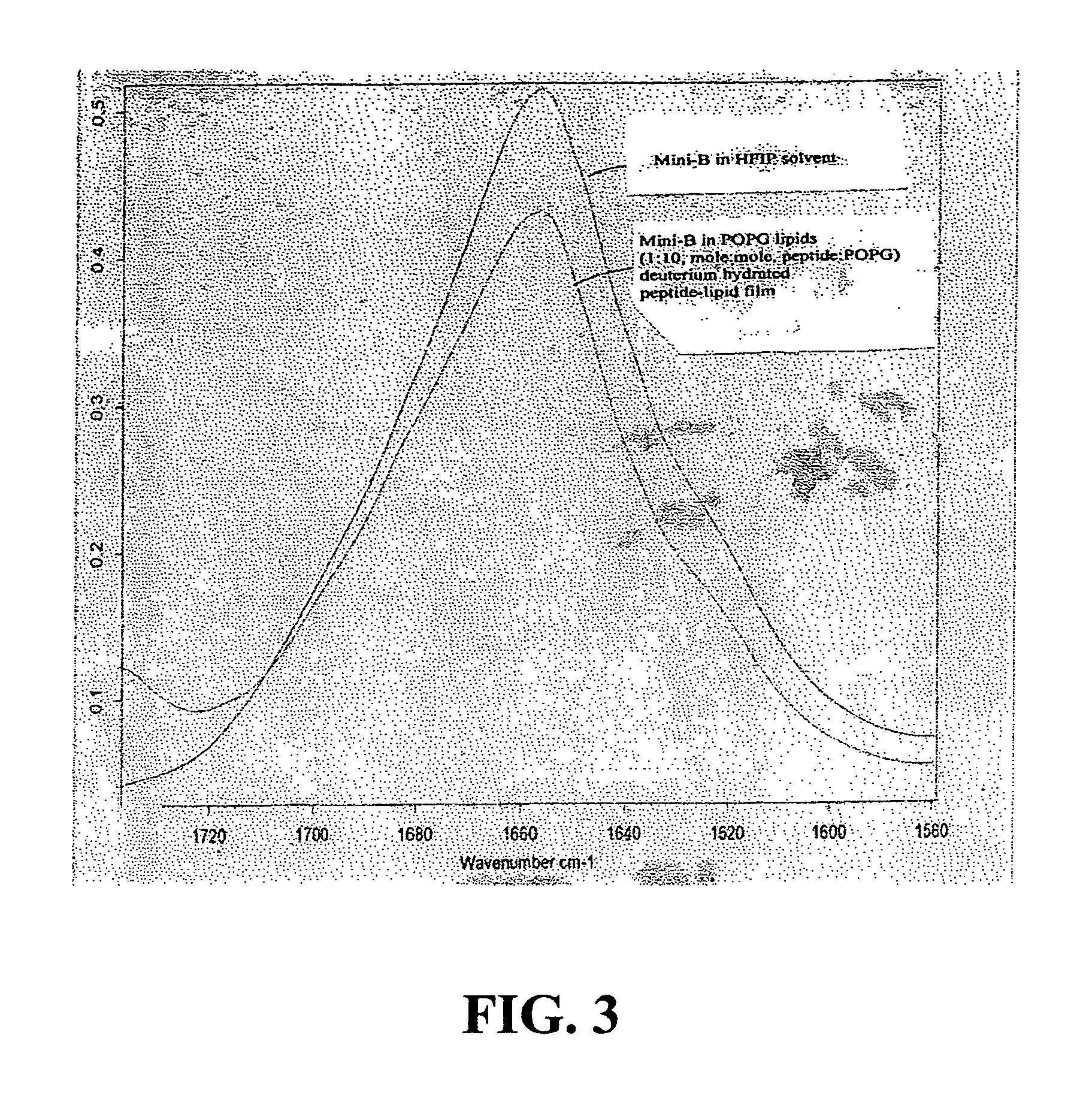
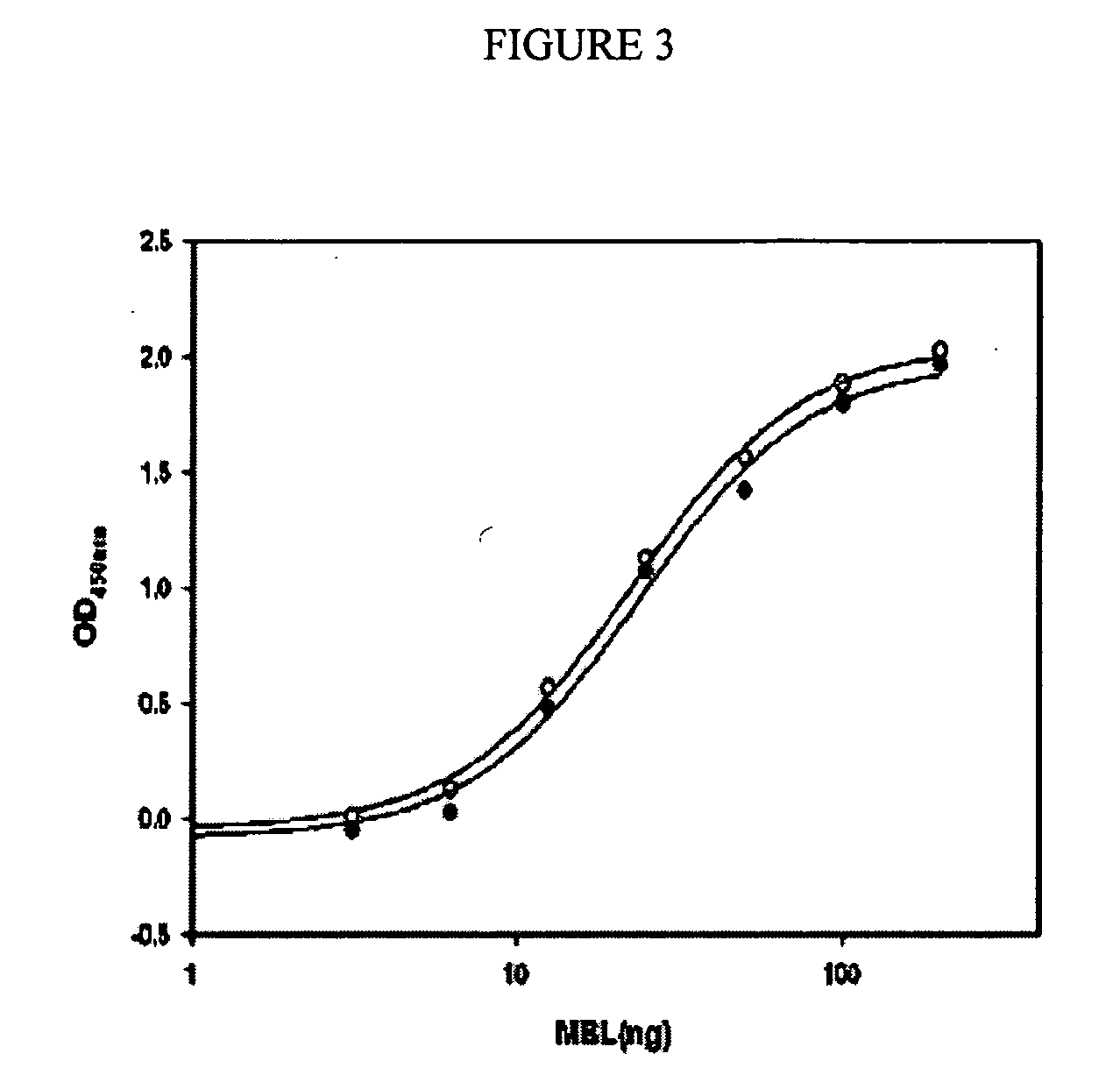
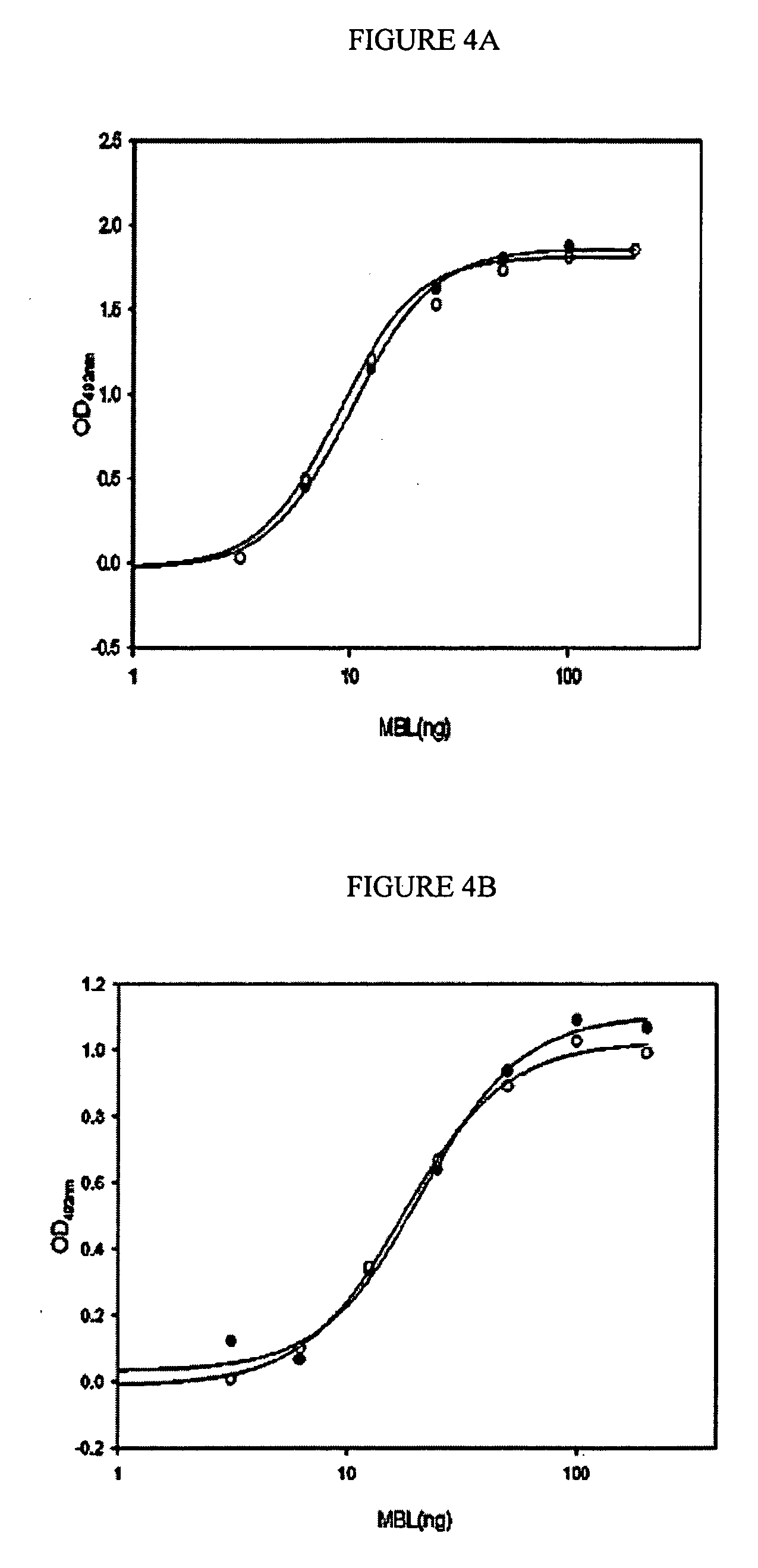
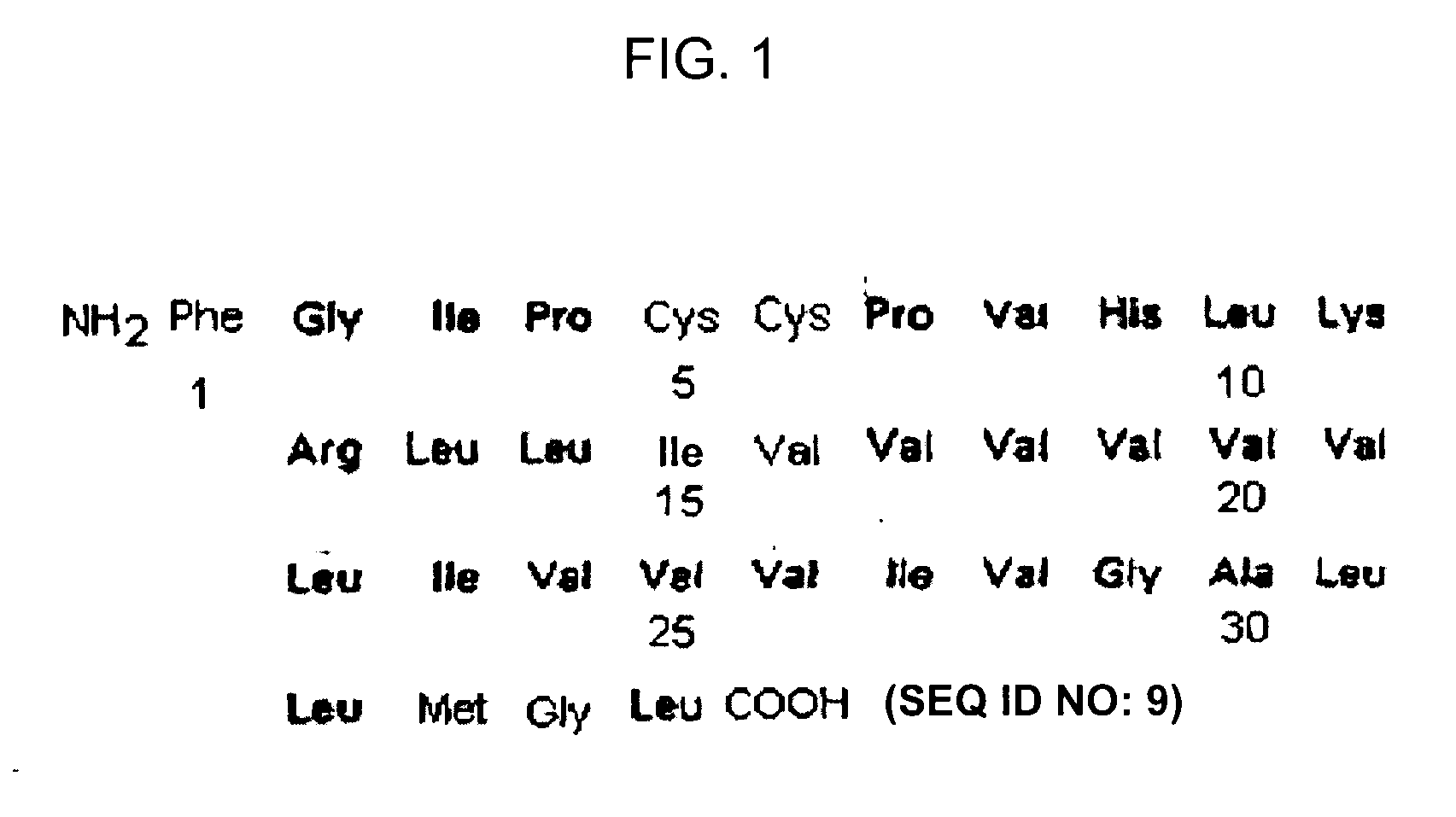
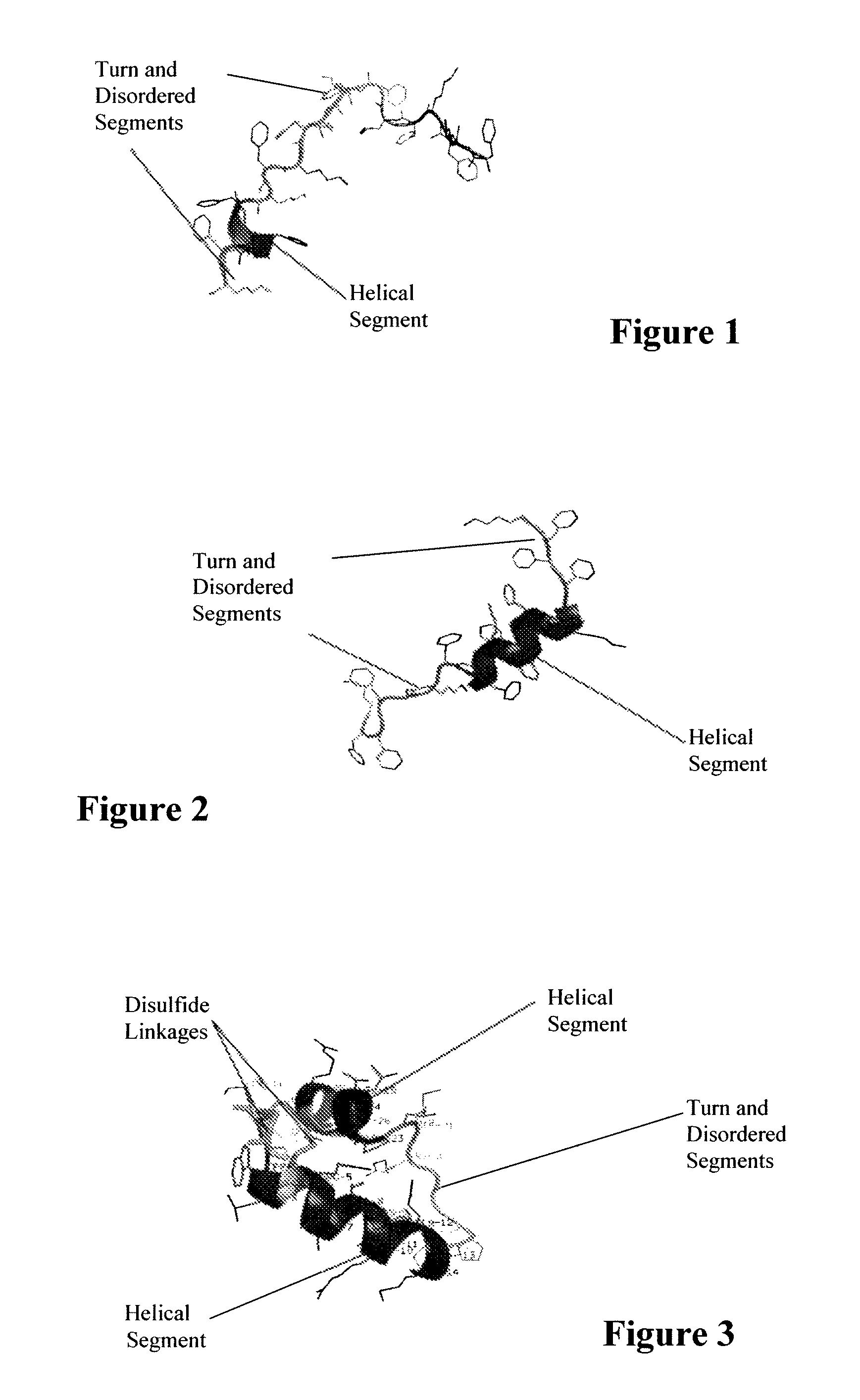
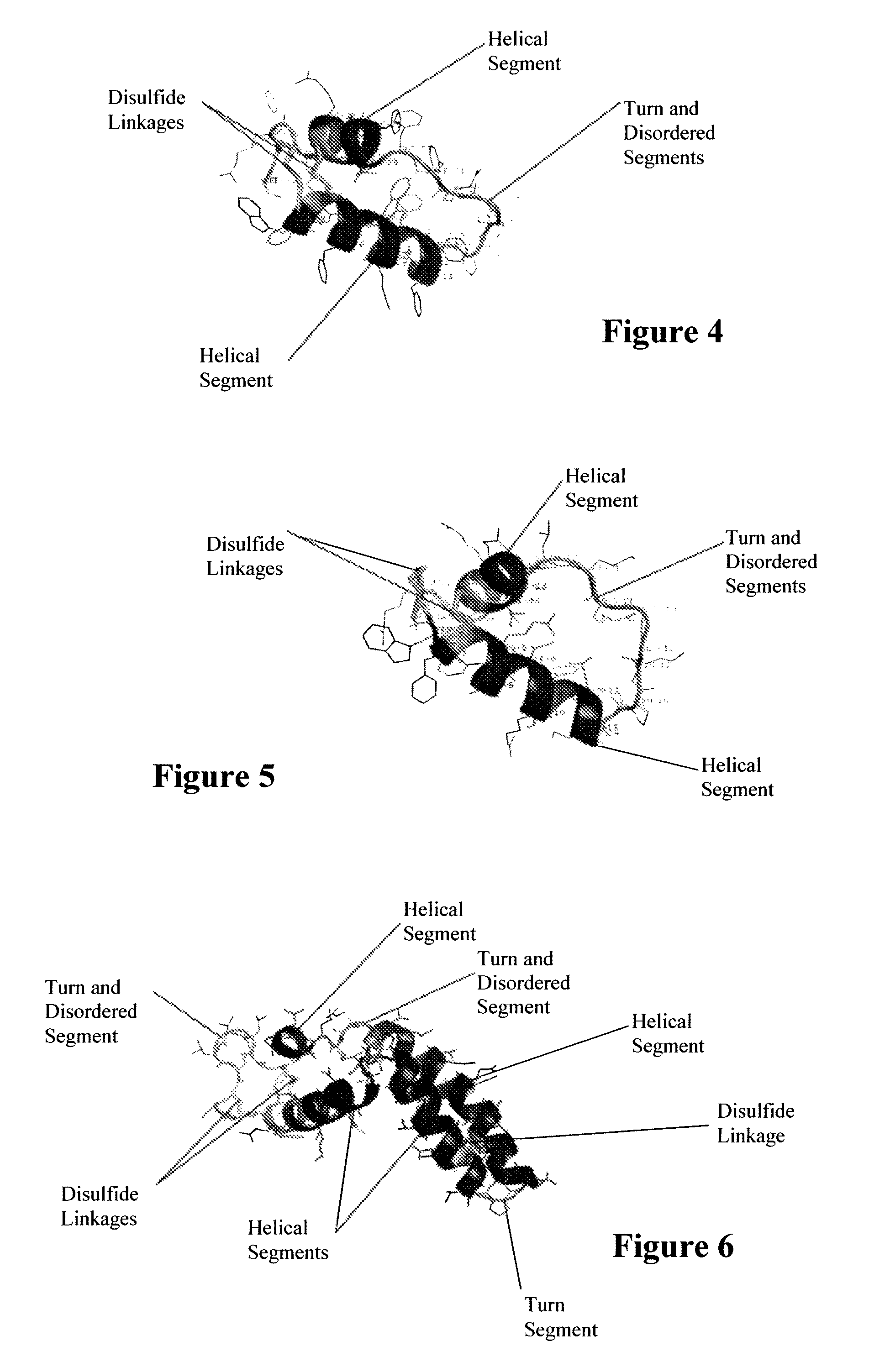
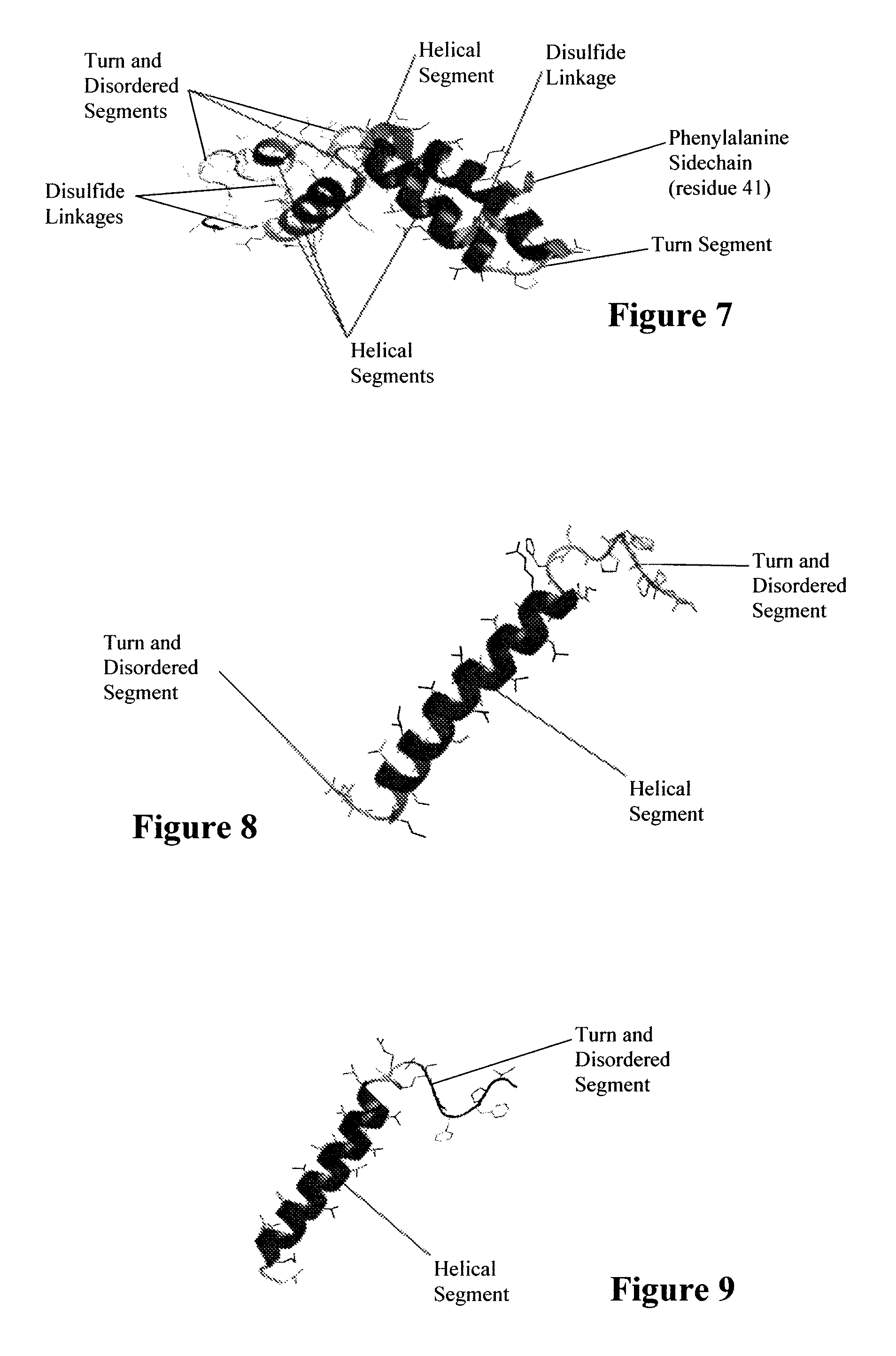
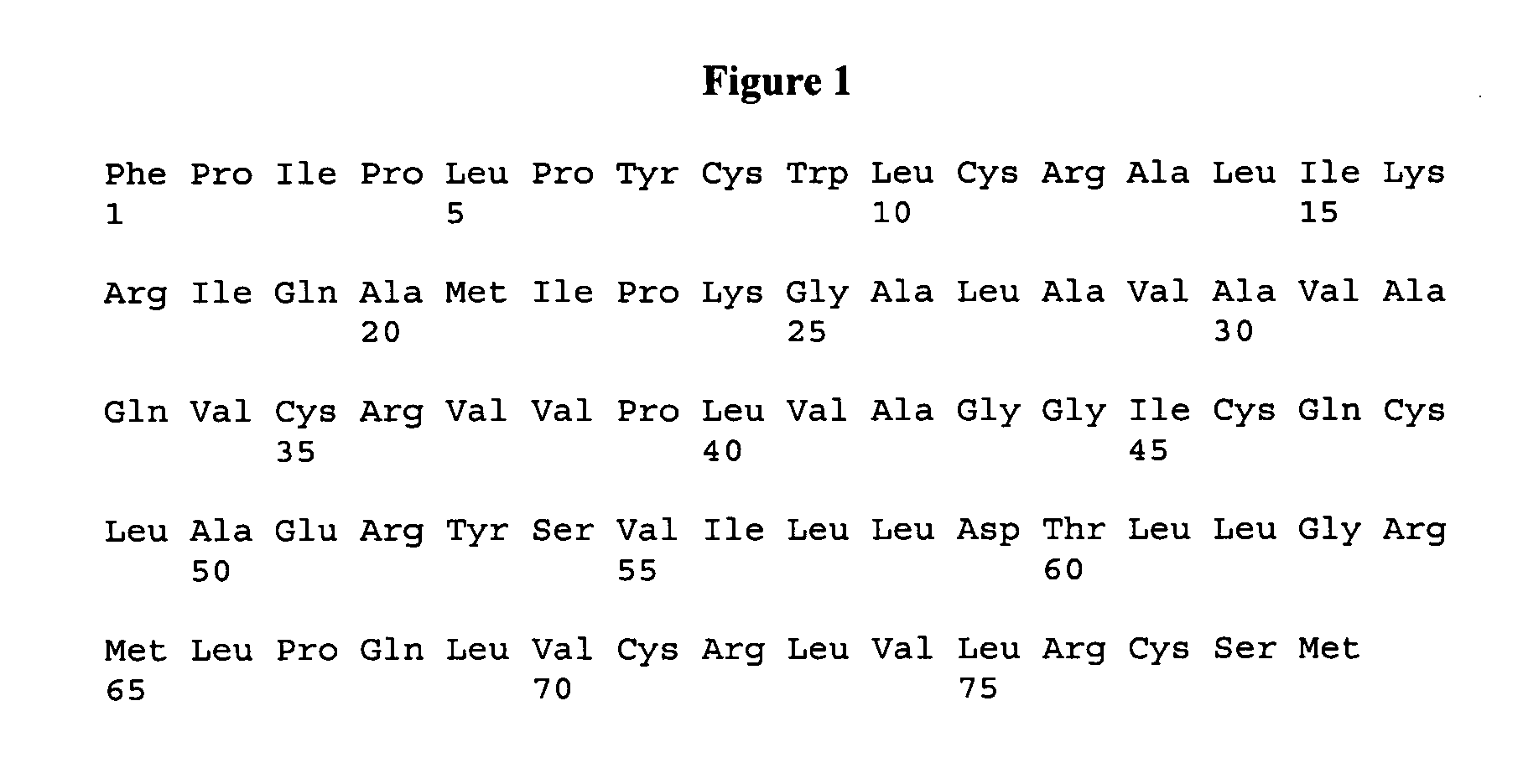
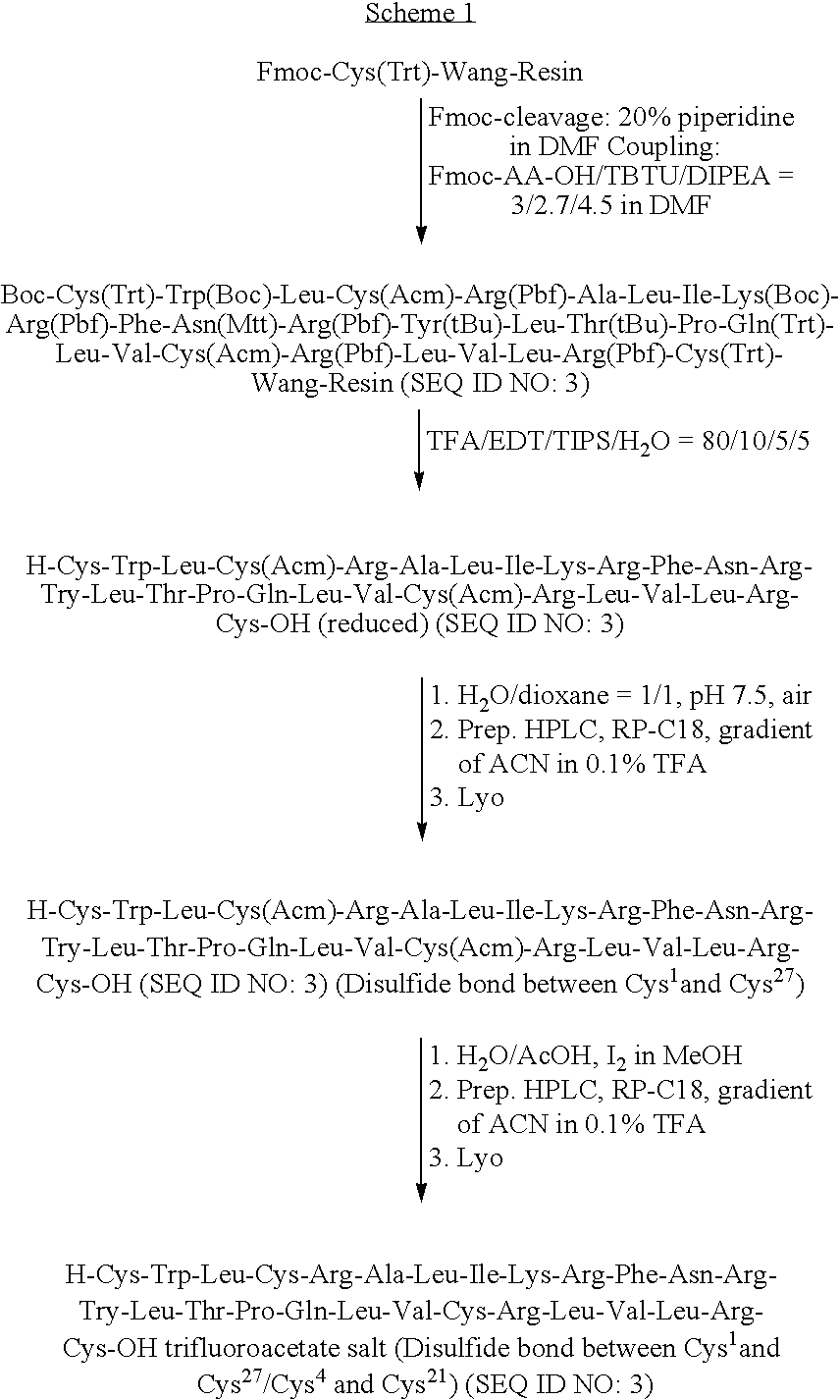
ActiveUS7538090B1Improve compliancePeptide/protein ingredientsAlveolar/pulmonary surfactant peptidesNaturally occurring surfactantsDisulfide Linkage
A composition including a C terminal region having residues corresponding to a peptide identified by PDB ID: 1RG3; an N terminal region having residues corresponding to a peptide identified by PDB ID: 1RG4; and a disulfide linkage between the residues near the C terminal region and the N terminal region. A composition including an exogenous peptide comprising amino acid residues comprising a C terminal region; amino acid residues comprising an N terminal region; a helix-loop-helix conformation between the residues comprising the C terminal region and the residues including the N terminal region; and at least one disulfide linkage between the residues comprising the C terminal region and the residues including N terminal region, wherein the residues including the C terminal region and the residues comprising the N terminal region have an amphiphatic property, and wherein the peptide has an a biological activity comparable to native surfactant protein SP-B. A method including delivering to a body a composition comprising an exogenous peptide having a biological activity comparable to native surfactant protein SP-B. A kit including an exogenous peptide having a biological activity comparable to native surfactant protein SP-B; and a treatment agent different from the peptide.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
Spray-dried collectin compositions and process for preparing the same
ActiveUS20070154406A1Excellent characteristicsImprove propertiesAntibacterial agentsPowder deliveryCollectinFungal microorganisms
The present invention relates to a spray-dried composition comprising as an active ingredient at least one member protein of the collectin family or its functional equivalent for treating and preventing microbial infectious diseases. The present invention also relates to a method for producing the same composition. The composition produced by the method of the present invention is effective in suppressing infections caused by viruses, bacteria, fungi, and parasites. Since the composition is developed in a form suitable for inhalation, it can directly provide the active ingredient to the sites of infection from these microbes, and thus treat and prevent respiratory infections and external wounds.
Owner:CHA VACCINE RES INST CO LTD
Surfactant peptide nanostructures, and uses thereof
InactiveUS7671258B2Improve efficiencyIncrease flexibilityPeptide-nucleic acidsMaterial nanotechnologyCrystallographyValine
Owner:MASSACHUSETTS INST OF TECH
Reconstituted surfactants having improved properties
ActiveUS20100004173A1Improving alveolar patencyMaintaining alveolar patencyPeptide/protein ingredientsAlveolar/pulmonary surfactant peptidesActive agentActive protein
Owner:CHIESI FARM SPA
Removal of lipopolysaccharides from protein-lipopolysaccharide complexes by non flammable solvents.
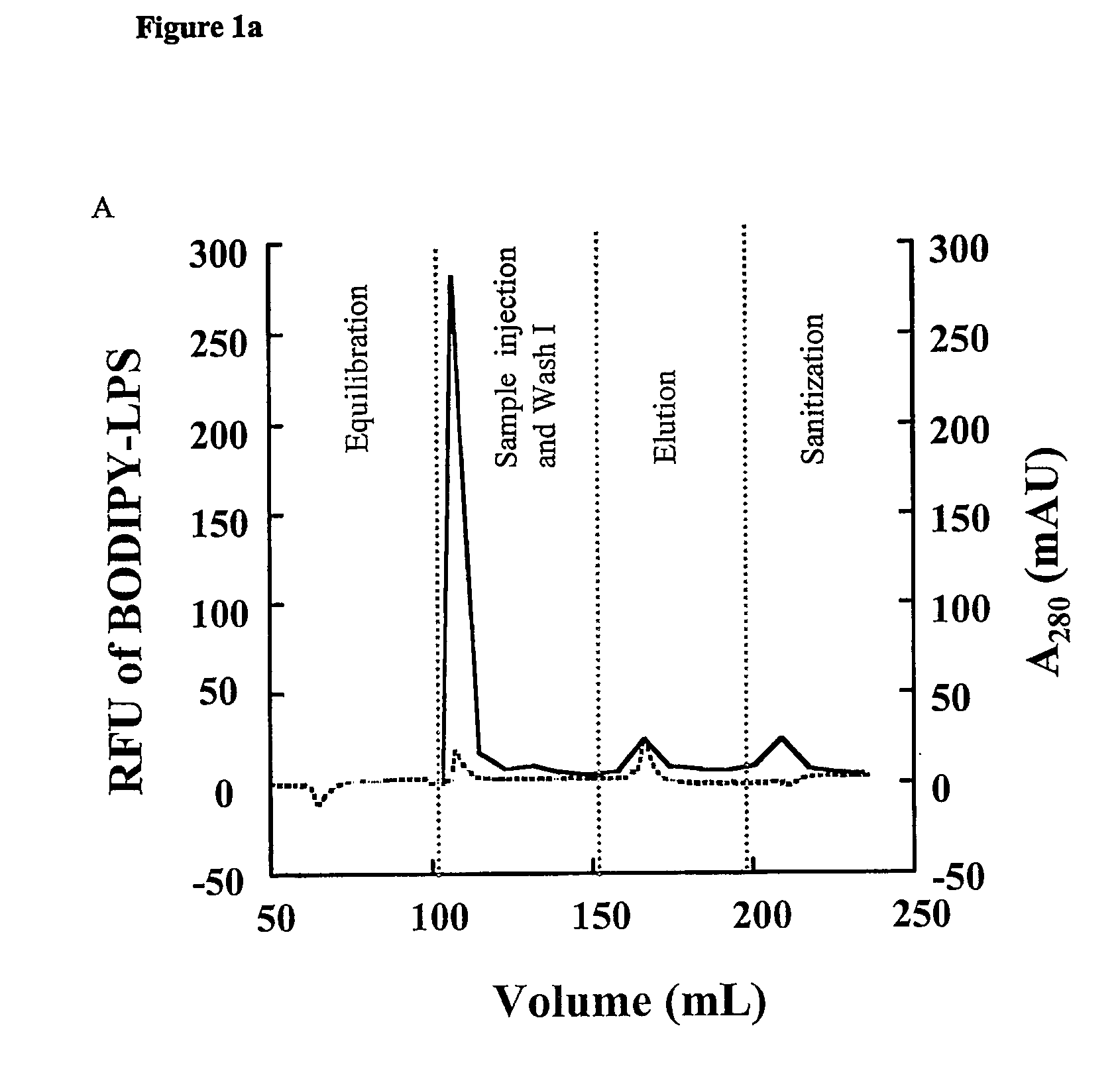
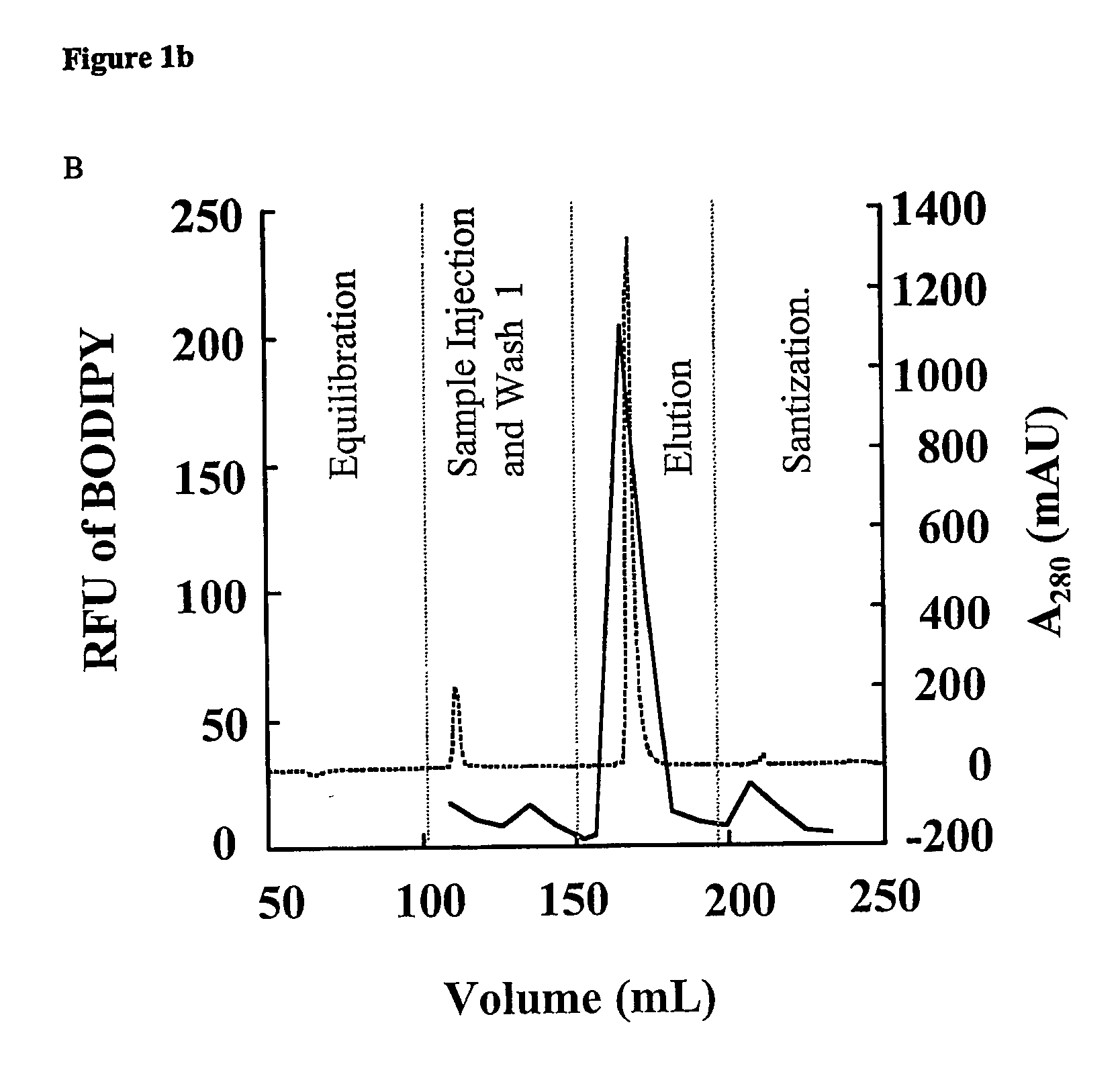
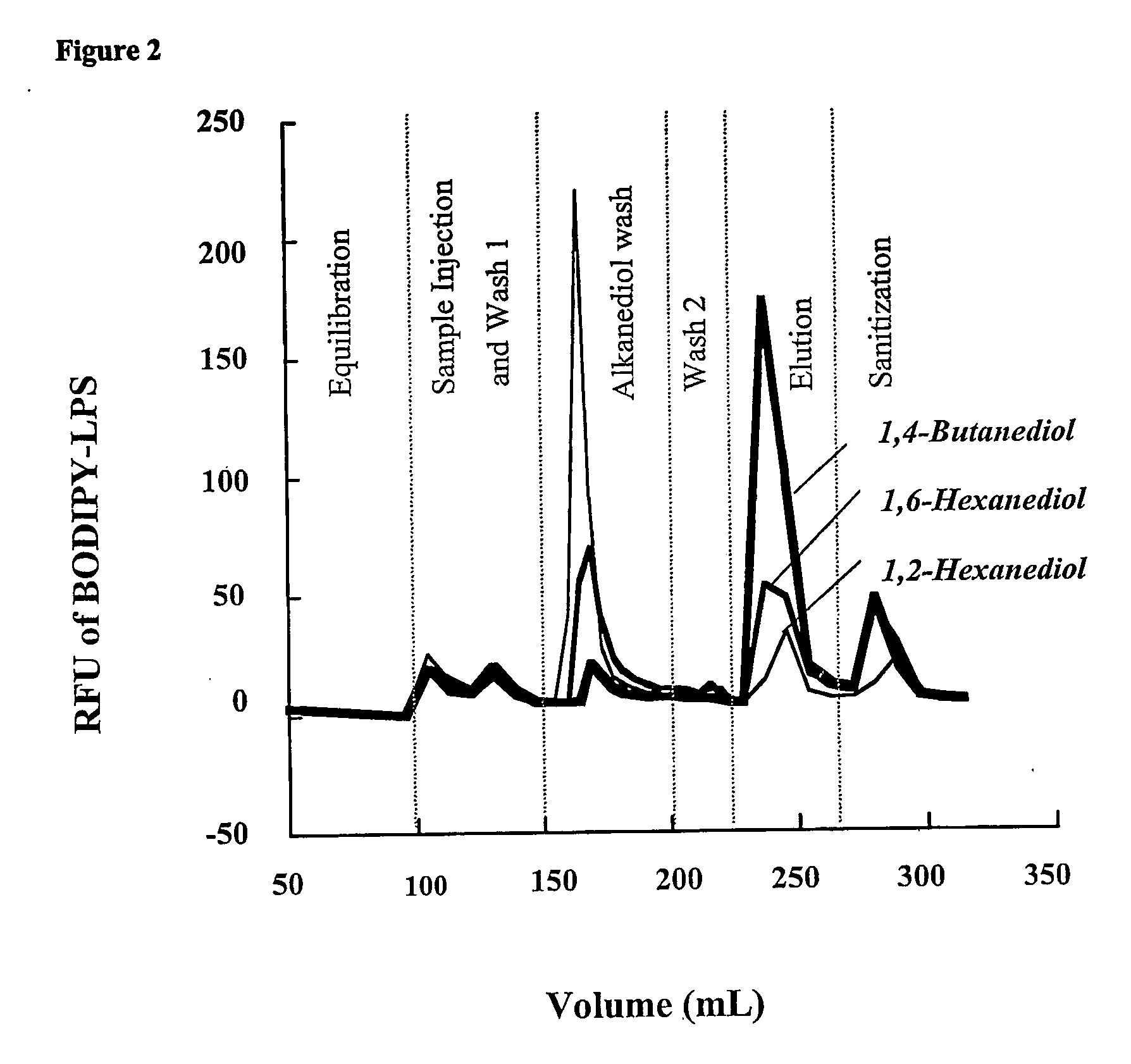
During the production of recombinant proteins from gram negative bacteria, lipopolysaccharides (LPS, endotoxin) are released along with the protein of interest. In many instances, LPS will copurify with the target protein due to specific or non-specific protein-ILPS interactions. We have investigated the ability of alkanediols to effect the separation of LPS from protein-LPS complexes while the complexes are immobilized on anion or cation exchange chromatographic media. Alkanediols provide a safer alternative to the use of other organics such as alcohols or acetonitrile due to their lower toxicity and decreased flammability. In addition, they are less costly than many of the detergents that have been used for such purposes. LPS removal efficiency increased with increasing alkane chain length. 1,2-alkanediols were more effective than terminal alkanediols in the separation of LPS from protein LPS complexes.
Owner:NV ORGANON
Compositions for treatment and prevention of pulmonary conditions
InactiveUS7863241B2Increase volumeDecreasing amountBiocidePeptide/protein ingredientsDiseasePhospholipase inhibitor
The invention provides compositions and methods for treating pulmonary conditions and for reducing the negative effects of pulmonary inflammation. Such compositions and methods employ protease inhibitors and a lung surfactant mixture. The compositions and methods can also include lipase inhibitors (e.g. a phospholipase inhibitors) and anti-oxidants.
Owner:THE SCRIPPS RES INST
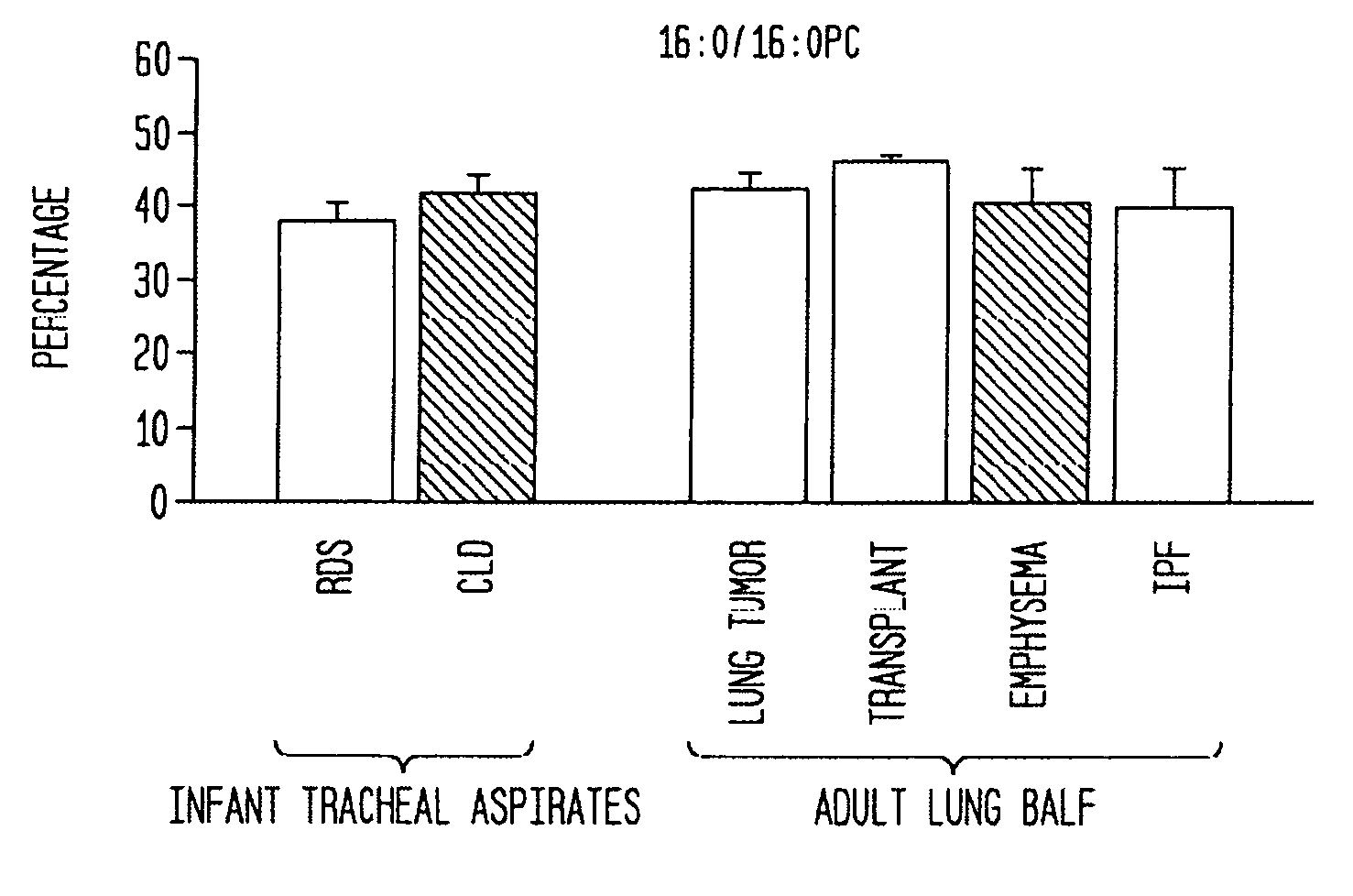
Phospholipid formulations and uses thereof in lung disease detection and treatment
InactiveUS20070010429A1Deter/inhibit damagePowder deliveryOrganic active ingredientsAir sacsSURFACTANT BLEND
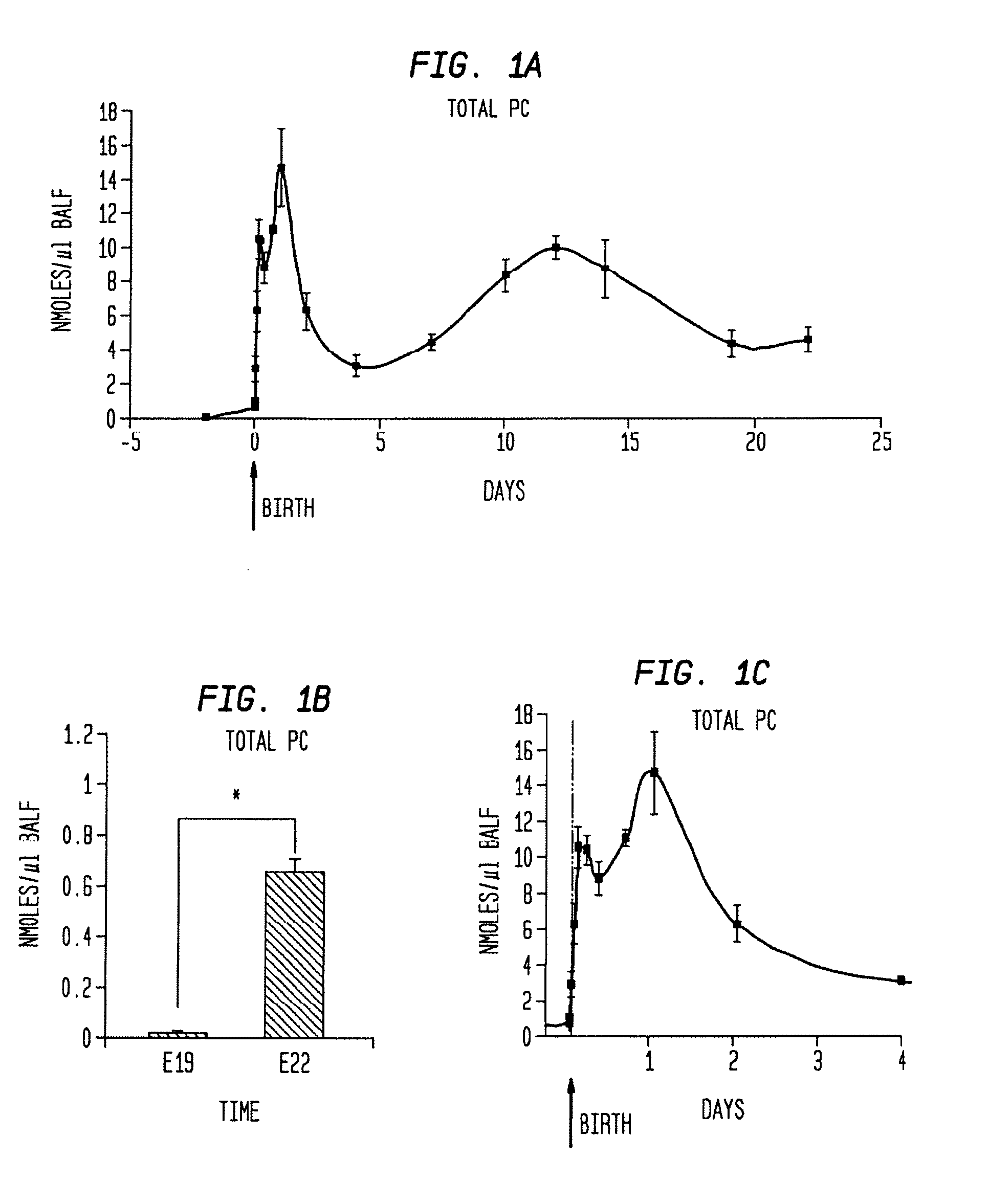
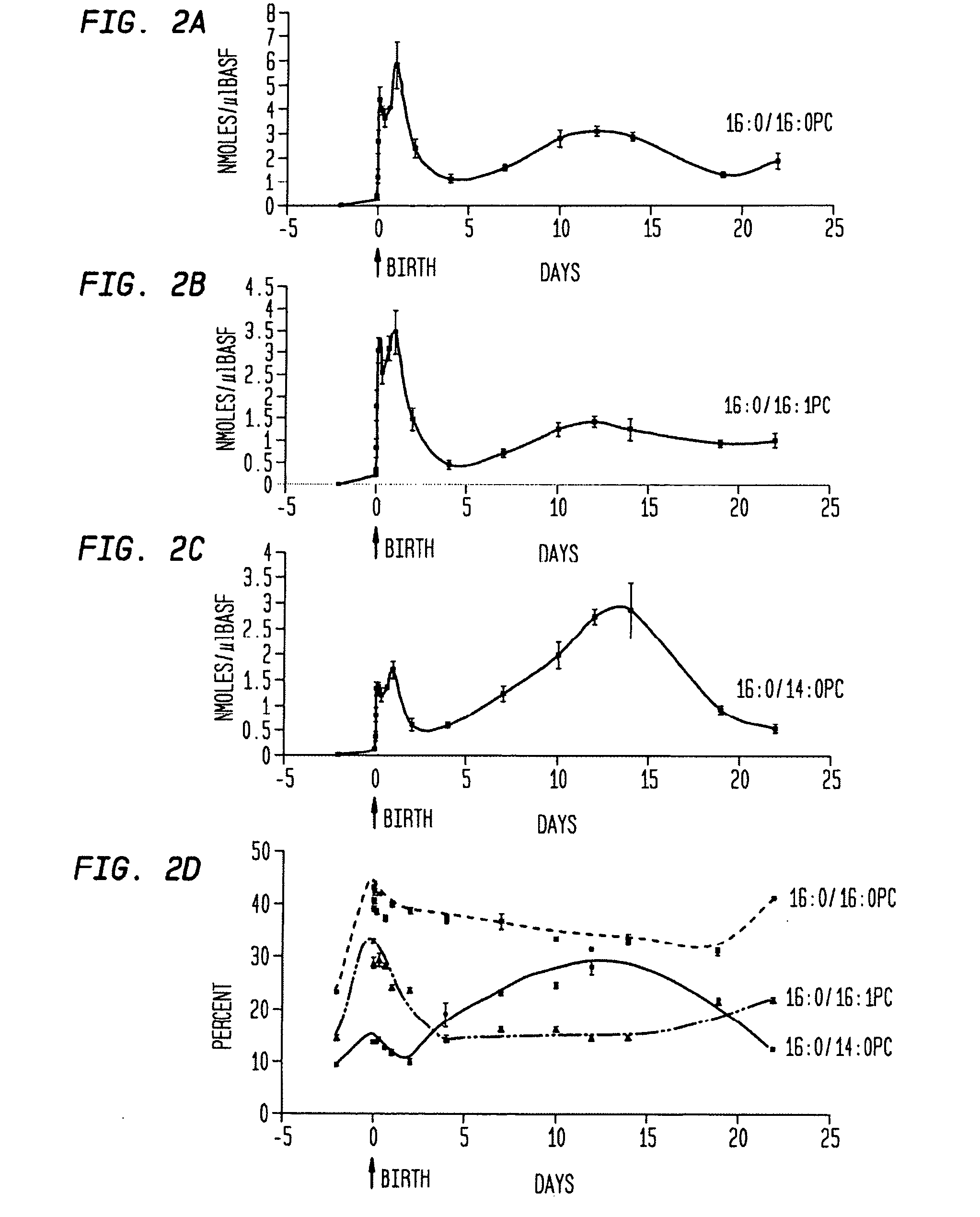
Disclosed are methods and compositions that are useful in the detection and therapy of diseases (e.g., emphysema) and damage that afflict the lungs. In some aspects, the compositions comprise a formulation enriched for a species of phosphatidylcholine, such as palmitoylmyristoyl phosphatidylcholine (16:0 / 14:0PC). The compositions may further be described as lung surfactant supplement preparations particularly useful in the treatment of pulmonary diseases and afflictions prevalent among premature infants, and in particular, Respiratory Distress Syndrome (RDS). A PC marker is also disclosed, 16:0 / 14:0PC, that may be used to detect pulmonary disease or reduced / compromised alveolar function in an animal. Phospholipid profiles of 16:0 / 14:0PC, 16:0 / 16:1PC and 16:0 / 16:0PC are also provided, and are correlated with particular pulmonary diseased states.
Owner:HOSPITAL FOR SICK CHILDREN
Fire extinguishing agent and fire extinguishing method using same
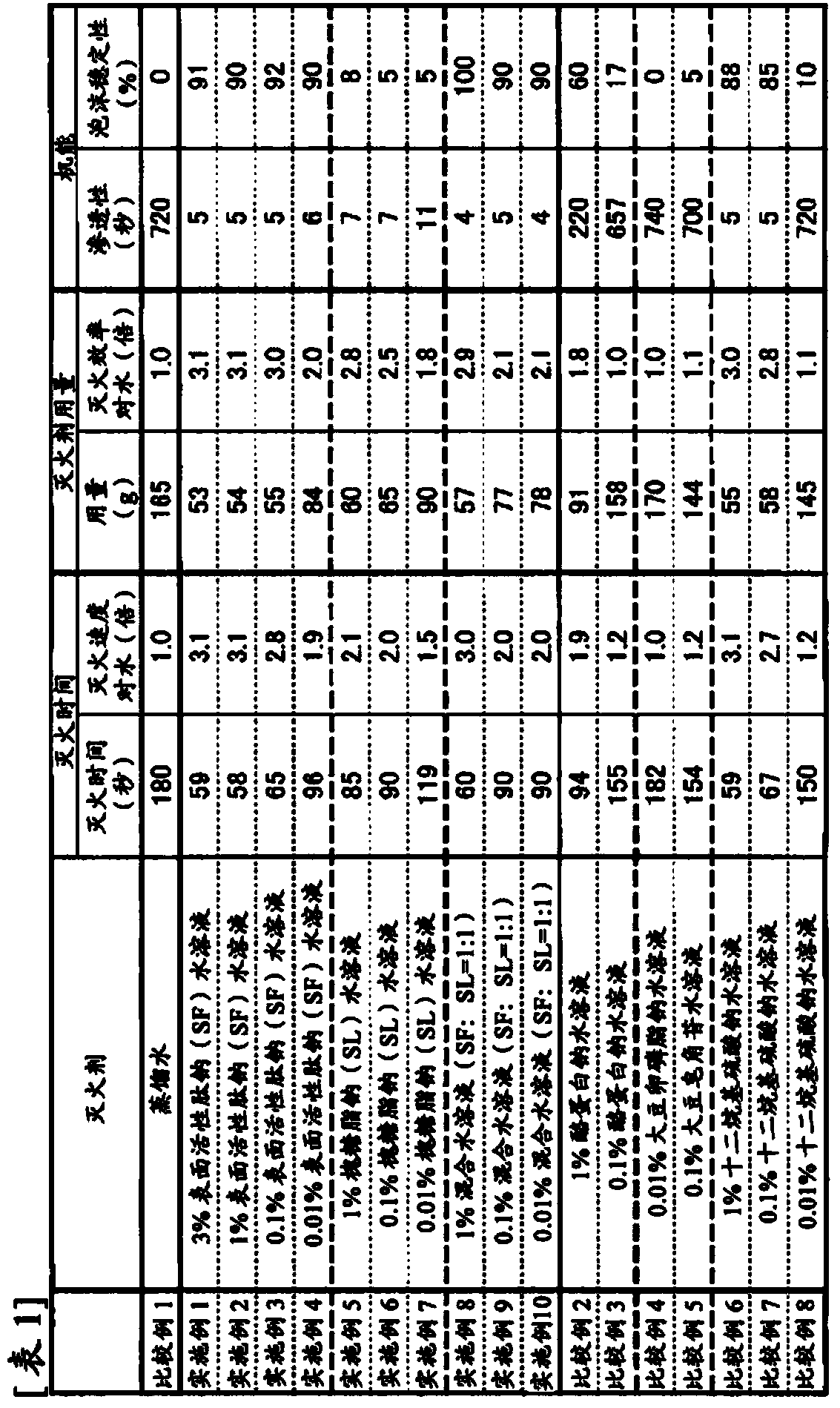
ActiveCN103702723AImproved fire extinguishing efficiencyReduce concentrationAlveolar/pulmonary surfactant peptidesFire extinguisherEngineering
The purpose of the invention is to provide a fire extinguishing agent which has both high fire-extinguishing performance and a high level of safety to the environment and the human body. This fire extinguishing agent is characterized by containing a biosurfactant.
Owner:KANEKA CORP
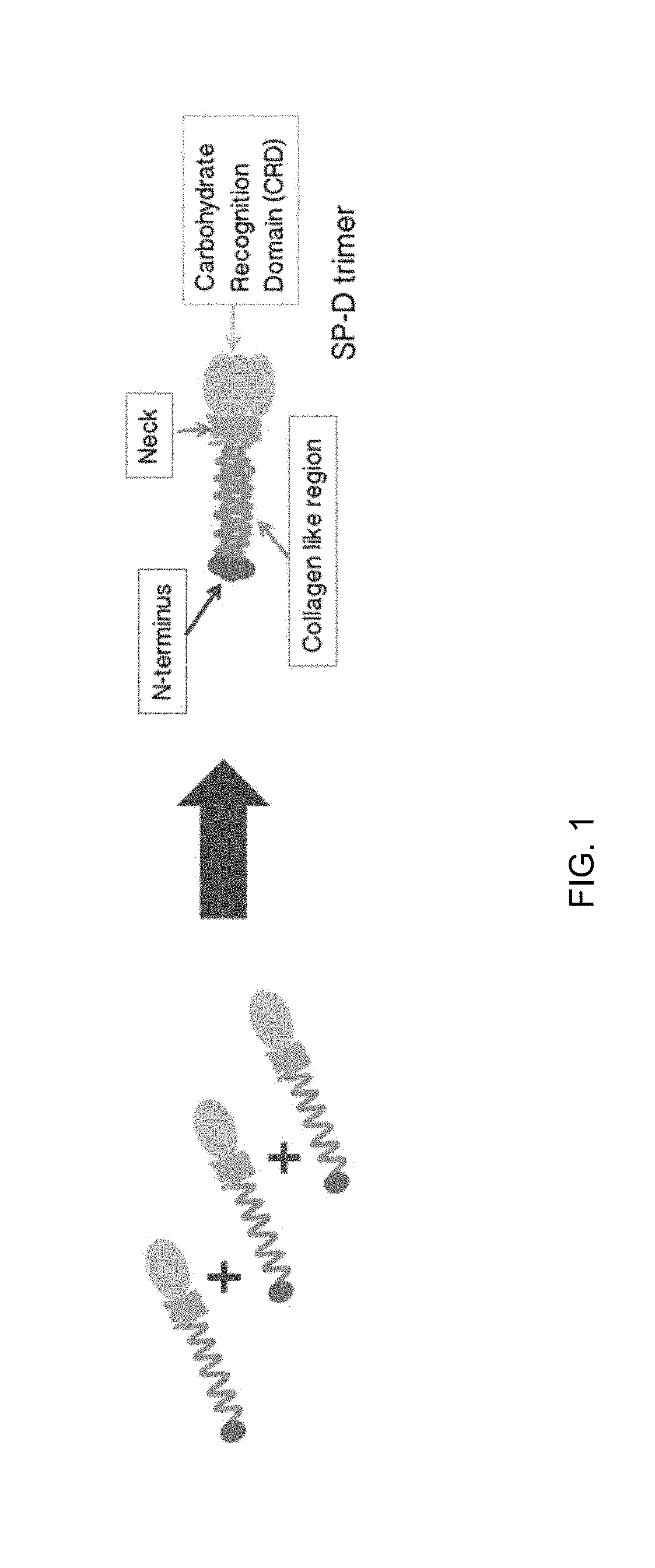
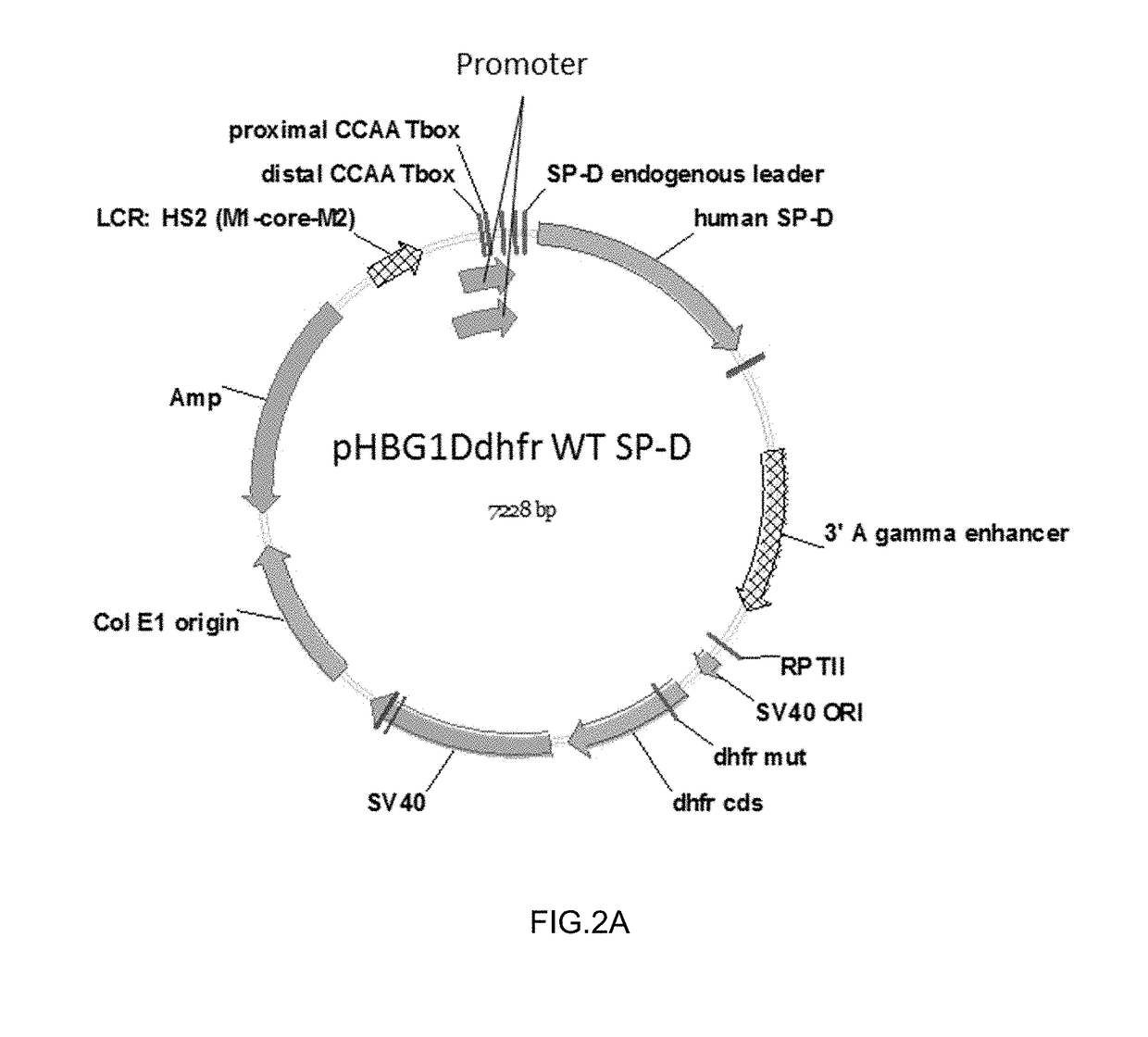
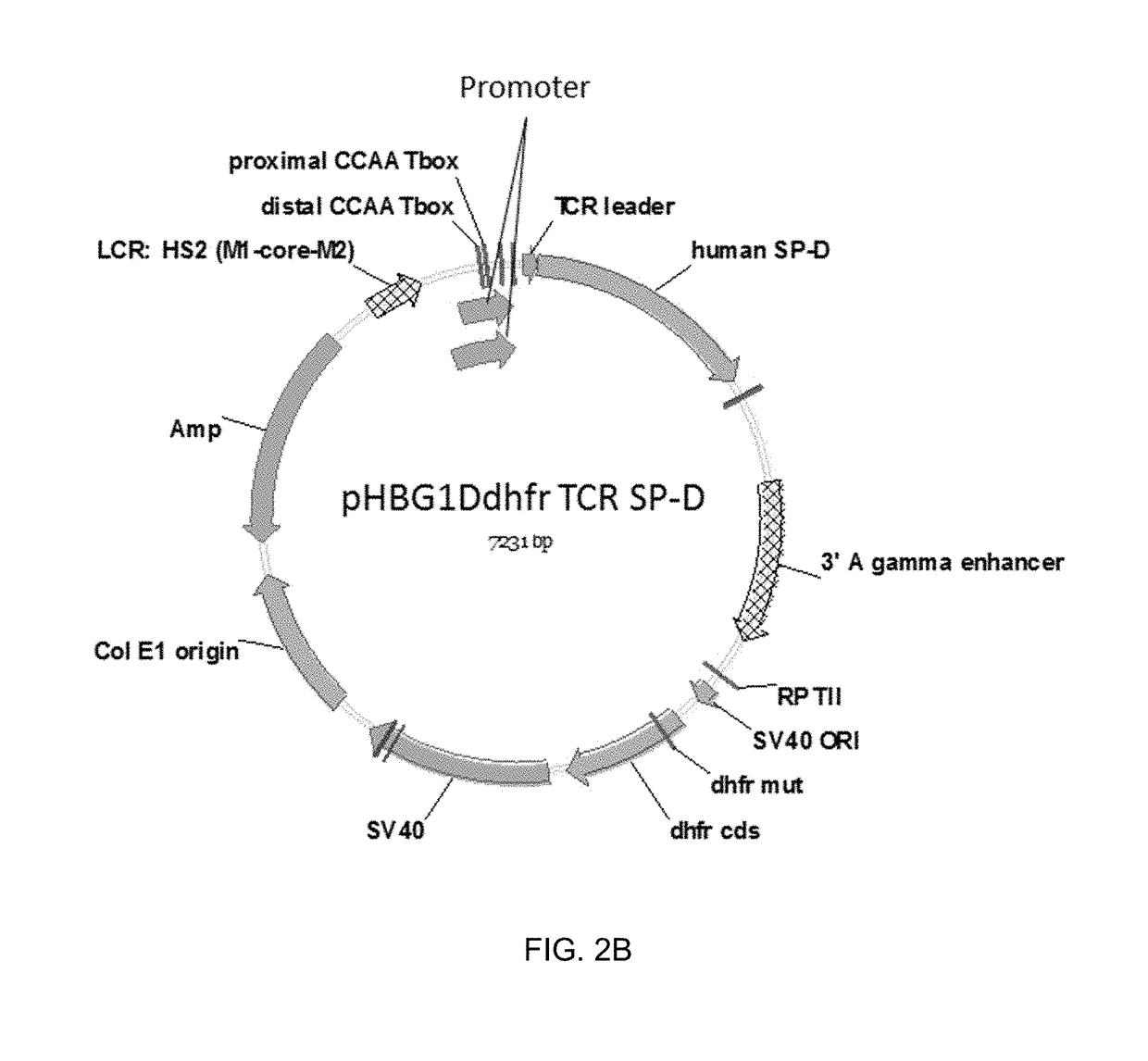
Methods, compositions and cells for preparing surfactant protein d (sp-d)
ActiveUS20190071693A1High expressionImprove concentrationImmunoglobulin superfamilyAntibody mimetics/scaffoldsSurfactant protein DProtein C
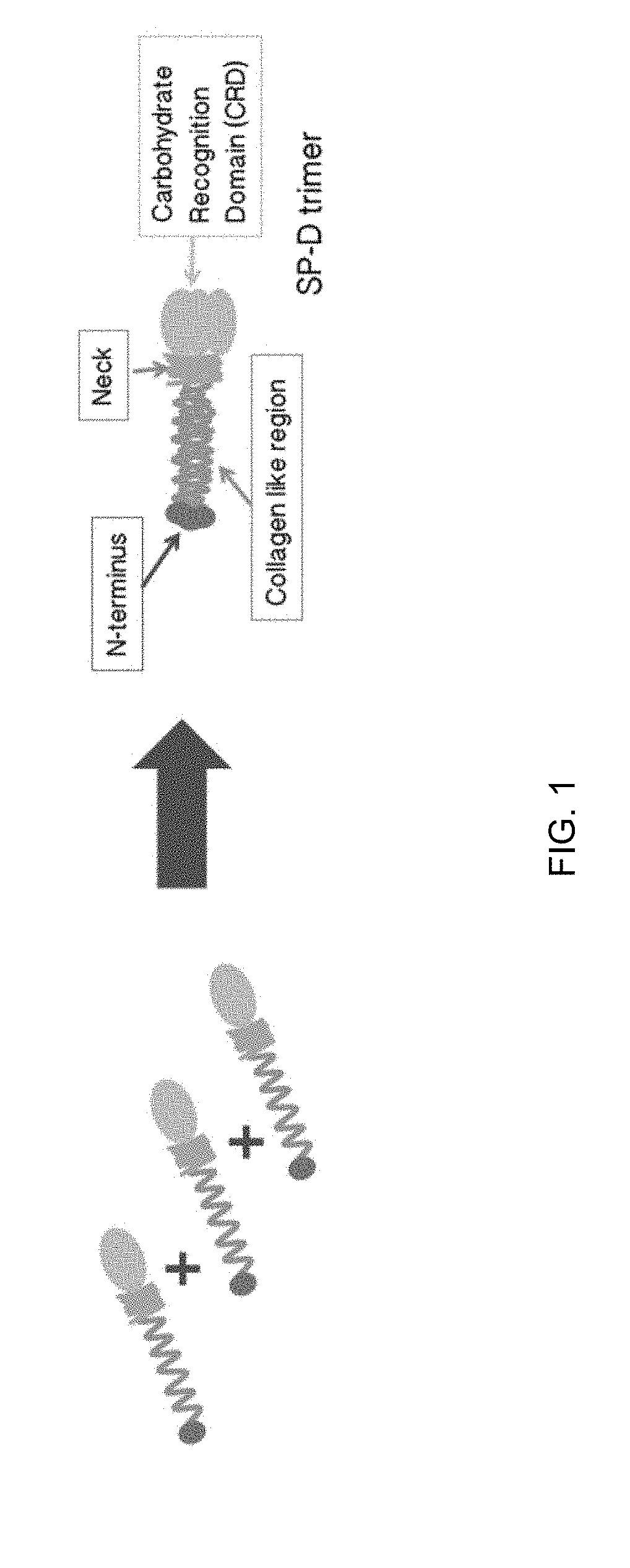
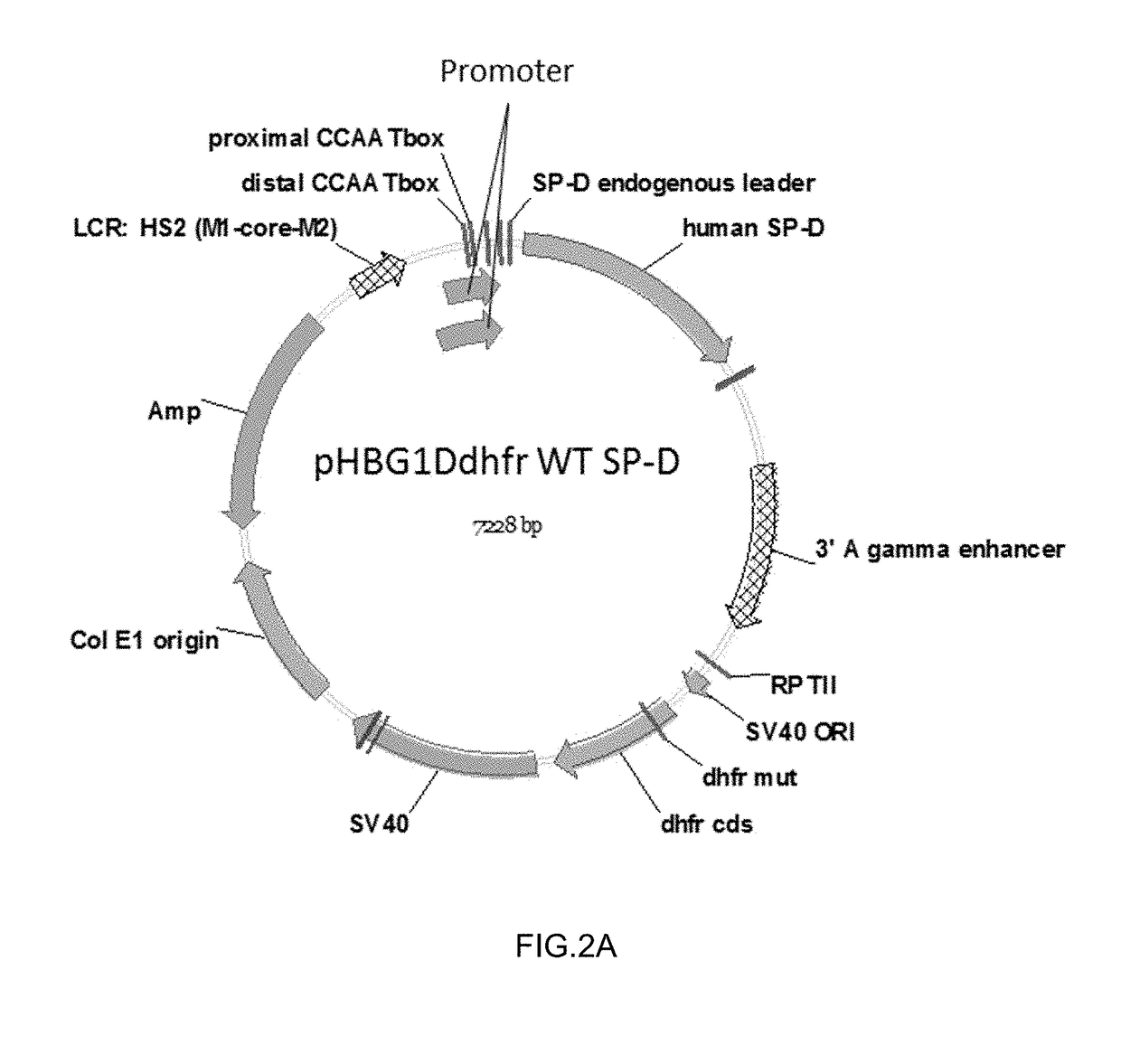
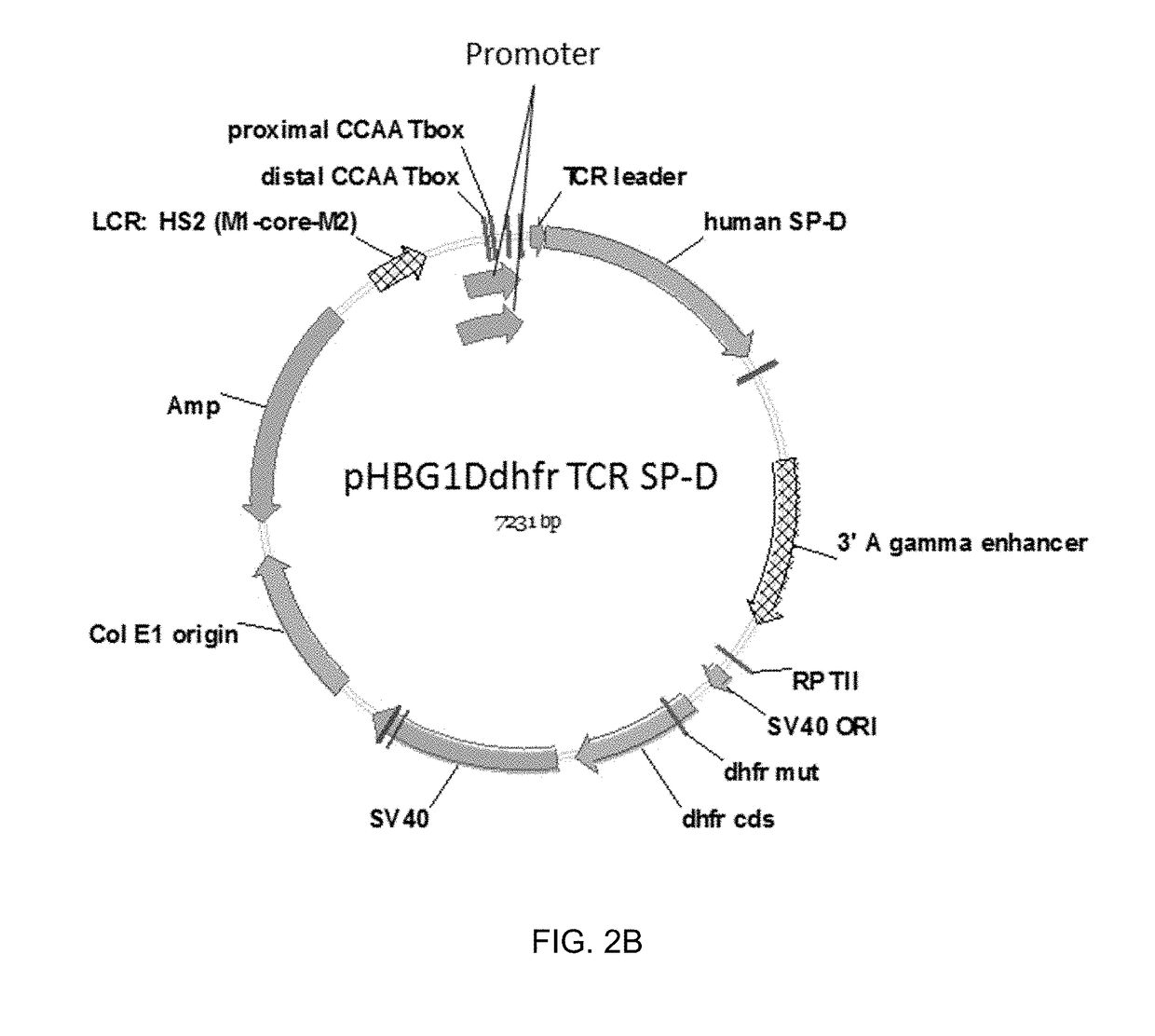
Some embodiments of the methods and compositions provided herein relate to the preparation surfactant protein-D (SP-D). Some embodiments include the expression of human SP-D in certain cell lines, and the purification of human SP-D from such cell lines. Some embodiments include the preparation of certain oligomeric forms of human SP-D.
Owner:AIRWAY THERAPEUTICS INC +1
Surfactant protein d for the treatment of disorders associated with lung injury
InactiveUS20110189104A1Symptoms improvedPrevention of pulmonary diseasePeptide/protein ingredientsAerosol deliveryCollectinDisease
Surfactant protein D (SP-D) is a 43-kDa member of the collectin family of collagenous lectin domain-containing proteins that is expressed in epithelial cells of the lung. Described herein are methods and compositions for the treatment of disorders associated with lung injury, including methods and compositions for the treatment of bronchopulmonary disorder (BPD) using recombinant human surfactant protein D and surfactant formulations.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Alkylated sp-b peptoid compounds and related lung surfactant compositions
InactiveUS20130065833A1Reduce surface tensionOrganic active ingredientsPeptide/protein ingredientsSurfactant protein ASurface-active agents
Owner:NORTHWESTERN UNIV
Methods and Compositions for Delivery of Medicaments to the Lungs
ActiveUS20120010145A1Reduce surface tensionProlong the action timePowder deliveryPeptide/protein ingredientsInhalationSurface-active agents
The disclosure provides a drug composition formulated for inhalation comprising a conjugate of a surface active agent and a pulmonary active drug. The surface active agent has an affinity for the human alveolar / gas interface and comprises at least a portion of a mammalian lung surfactant of a mimic thereof. The disclosure also provides a method of treating a subject suffering from or at risk of suffering from a lung disease comprising administering to the subject a conjugate comprising a drug for lung treatment and a surface active agent by inhalation in an amount effective to induce a drug effect in the lungs.
Owner:PAKA PULMONARY PHARMA
Reconstituted pulmonary surfactants
ActiveUS20140142021A1Improve compliancePeptide/protein ingredientsAlveolar/pulmonary surfactant peptidesDiseaseMedicine
The present invention is directed to a reconstituted surfactant comprising a phospholipid mixture, and a combination of particular analogues of the native surfactant protein SP-C with analogues of the native surfactant protein SP-B. The invention is also directed to pharmaceutical compositions and kits thereof and to its use for the treatment or prophylaxis of RDS and other respiratory disorders.
Owner:CHIESI FARM SPA
Synthetic lipid mixtures for the preparation of a reconstituted surfactant
InactiveUS20080242589A1Peptide/protein ingredientsAlveolar/pulmonary surfactant peptidesDiseaseLipid formation
The invention relates to reconstituted surfactants consisting of artificial phospholipids and peptides able to lower the air-liquid surface tension, more particularly to reconstituted surfactants comprising special phospholipid mixtures and artificial peptides which are analogues of the natural surfactant SP-C protein for the treatment of respiratory distress syndrome (RDS) and other diseases relating to pulmonary surfactant dysfunctions.
Owner:CHIESI FARM SPA
Methods and compositions for preparing surfactant protein d (sp-d)
ActiveUS20190071694A1High expressionImprove concentrationAlveolar/pulmonary surfactant peptidesPeptide preparation methodsSurfactant protein DProtein C
Some embodiments of the methods and compositions provided herein relate to the preparation surfactant protein-D (SP-D). Some embodiments include the expression of human SP-D in certain cell lines, and the purification of human SP-D from such cell lines. Some embodiments include the preparation of certain oligomeric forms of human SP-D.
Owner:AIRWAY THERAPEUTICS INC
Reconstituted surfactants having improved properties
ActiveUS20090088379A1Peptide/protein ingredientsAlveolar/pulmonary surfactant peptidesSURFACTANT BLENDRepeat unit
The present invention is directed to a reconstituted surfactant containing a lipid carrier, a polypeptide analog of the native surfactant protein SP-C, and a polypeptide comprising a sequence comprised of repeated units where each unit contains between 3 and 8 hydrophobic amino acid residues and one basic amino acid residue. The invention is also directed to the pharmaceutical compositions thereof and to its use for the prophylaxis and / or treatment of RDS and other respiratory disorders.
Owner:CHIESI FARM SPA
Synthetic lung surfactant and use thereof
ActiveUS8563683B2Improve surface activityReduce surface tensionBiocidePeptide/protein ingredientsGlycerol DerivativesPhospholipase
The present invention relates to synthetic lung surfactant compositions that contain one or more of phospholipase-resistant phospho-glycerol derivatives, phospholipase-resistant phospho-choline derivatives, and surface active proteins or peptides, more preferably a combination of at least two or all three of these materials. Novel phospholipase-resistant phospho-glycerol derivatives, phospholipase-resistant phospho-choline derivatives, and surface active peptides are also disclosed herein. Uses of the surfactant compositions of the present invention to treat endogenous surfactant dysfunctional or deficient lung tissue, to prepare synthetic peptides for use in the surfactant compositions, and to deliver therapeutic agents are also disclosed.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT +2
Synthetic pulmonary surfactant peptides
ActiveUS20090075892A1Peptide/protein ingredientsAlveolar/pulmonary surfactant peptidesDiseaseLipid formation
The present invention is directed to a reconstituted surfactant comprising a lipid carrier, a polypeptide analog of the native surfactant protein SP-C, and a polypeptide analog of the native surfactant protein SP-B. The invention is also directed to the pharmaceutical compositions thereof and to a use thereof in the treatment or prophylaxis of RDS and other respiratory disorders.
Owner:CHIESI FARM SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com