Lamotrigine oral liquid preparation and preparation method thereof
A lamotrigine oral liquid, lamotrigine technology, which is applied in the directions of pharmaceutical formulations, drug delivery, nervous system diseases, etc. Facilitates absorption and improves bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 (oral liquid)
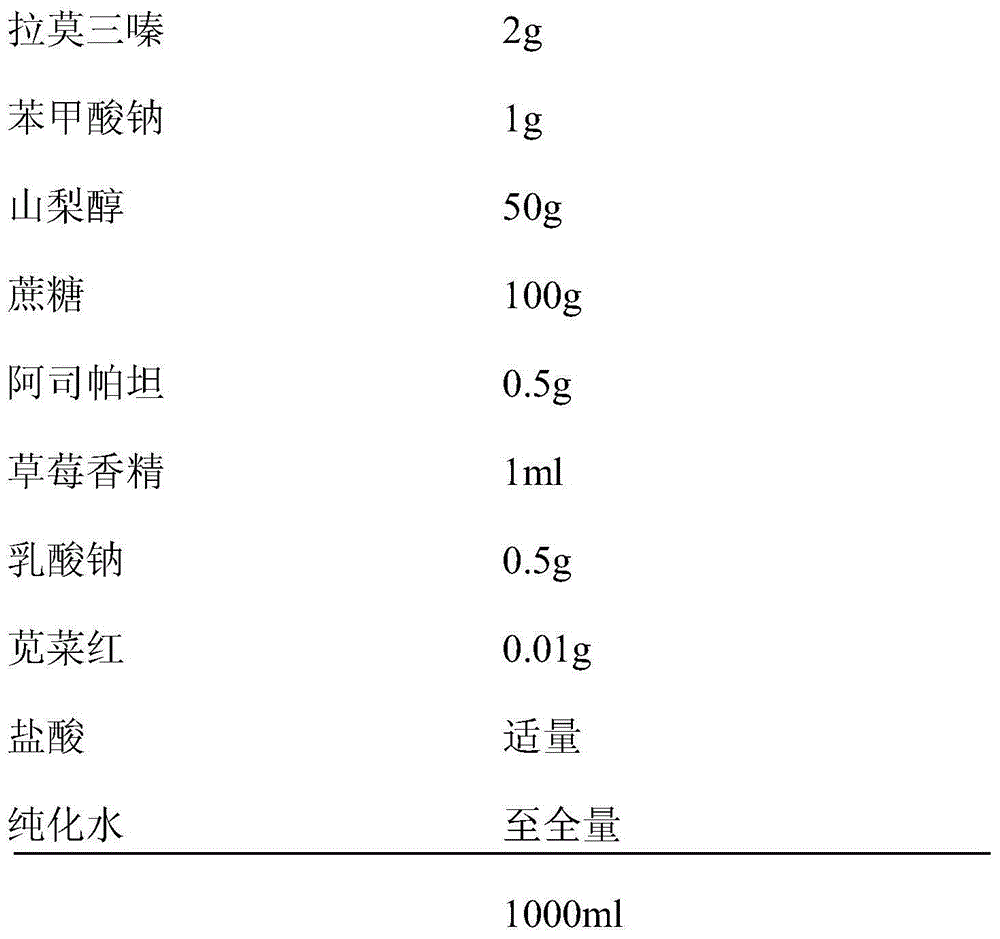
[0033]
[0034] The preparation method of the oral liquid preparation of lamotrigine in the present embodiment 1 is specifically implemented according to the following steps: Weigh the following components: lamotrigine 2g, sodium benzoate 1g, sorbitol 50g, sucrose 100g, aspartame 0.5 g, 1ml of strawberry essence, 0.5g of sodium lactate, 0.01g of amaranth, appropriate amount of hydrochloric acid, and the balance is purified water. Add sorbitol and lamotrigine, disperse evenly with a small amount of hot water, add to the liquid preparation tank, heat and boil for a period of time, cool, and stir for 10 minutes; mix the weighed sodium lactate, water-soluble sucrose and Spartan and amaranth are respectively dissolved in a small amount of warm water at 50°C and added to the liquid mixing tank, add fragrance, add purified water and stir; adjust the pH value to 2.5-4.0 with pH regulator hydrochloric acid, and then add purified water Dilute to f...
Embodiment 2
[0035] Embodiment 2 (syrup)
[0036]
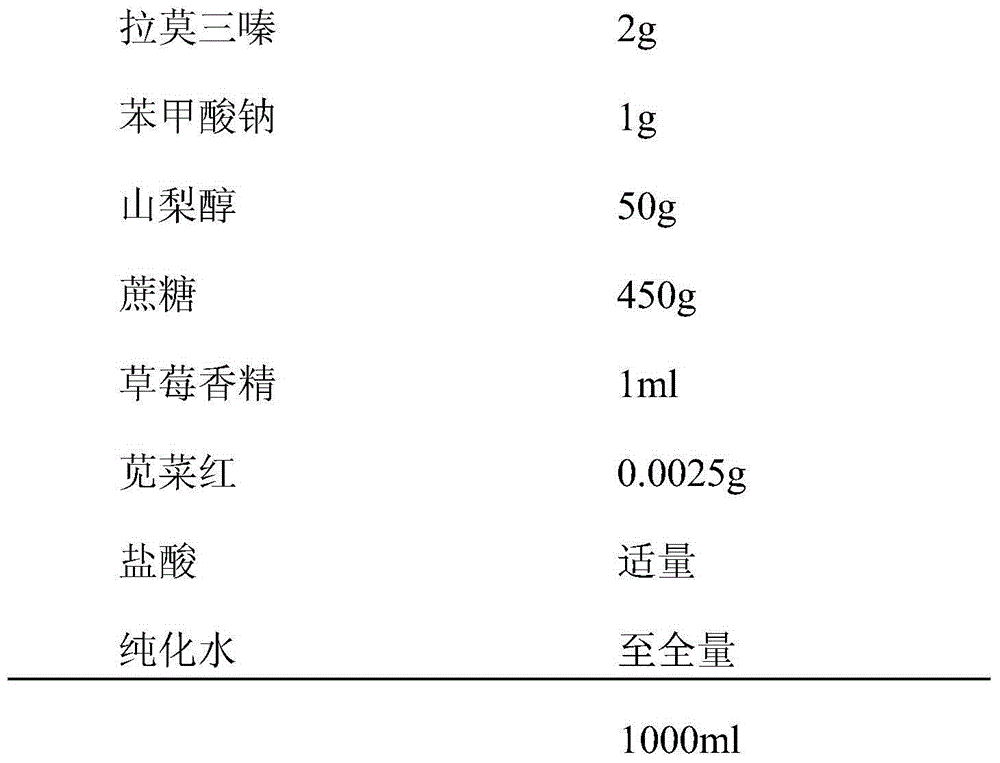
[0037]The preparation method of the oral liquid preparation of lamotrigine in the present embodiment 2 is specifically implemented according to the following steps: Weigh the following components: 2 g of lamotrigine, 1 g of sodium benzoate, 50 g of sorbitol, 450 g of sucrose, 1 ml of strawberry essence, and amaranth Red 0.0025g, appropriate amount of hydrochloric acid, the balance is purified water, the total amount of the above is 1000ml; take an appropriate amount of purified water and put it in a liquid mixing tank, heat to 40°C, add sucrose and sorbitol under stirring conditions, heat and boil until completely dissolved, Standby; put the weighed lamotrigine in a small beaker, dissolve it completely with hot water, and add it to the liquid preparation tank; add the weighed sodium benzoate, strawberry essence, and amaranth into the liquid preparation tank respectively , add purified water, and stir for 3 minutes; add the weighed pH r...
example
[0039] 1. Li, 8 years old, was clinically diagnosed with epilepsy with a medical history of 5 years. He had taken various anti-epileptic drugs and could not effectively control the seizures. While taking sodium valproate, add the composition of Example 1 of the present invention, the initial dose is 25 mg, take it every other day, and take it for two consecutive weeks; then once a day for two weeks, 25 mg each time. Thereafter, the dose is increased every 1-2 weeks, with a maximum increase of 25-50 mg. After 3 months, the drowsiness, headache and dizziness of the patient were basically eliminated, the taste was good, the swallowing property was good, and the medication compliance was good.
[0040] 2. Yan, 12 years old, was clinically diagnosed with epilepsy, with a medical history of 5 years and about 11 seizures per month. Taking the composition of Example 2 of the present invention, using this product as monotherapy, the initial dose is 25 mg, once a day, for two weeks; th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com