Piclofenac potassium sustained release capsule and preparing technique thereof
A technology of diclofenac potassium and sustained-release capsules, applied in the field of medicine, can solve the problems of unstable blood drug concentration, inconvenience to patients, toxic and side effects, etc., and achieve the effects of excellent release curve, reduced administration times, and low production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
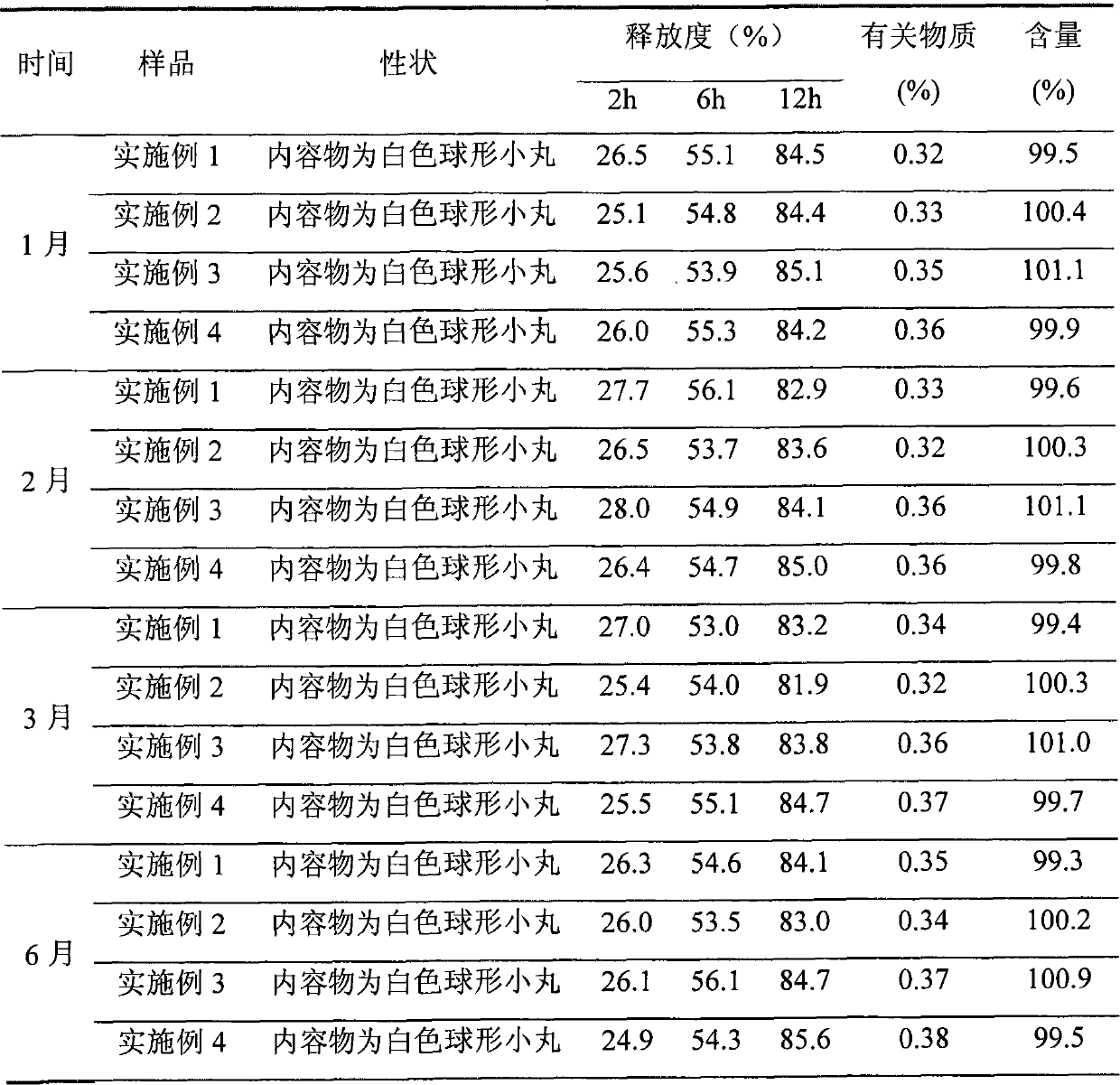
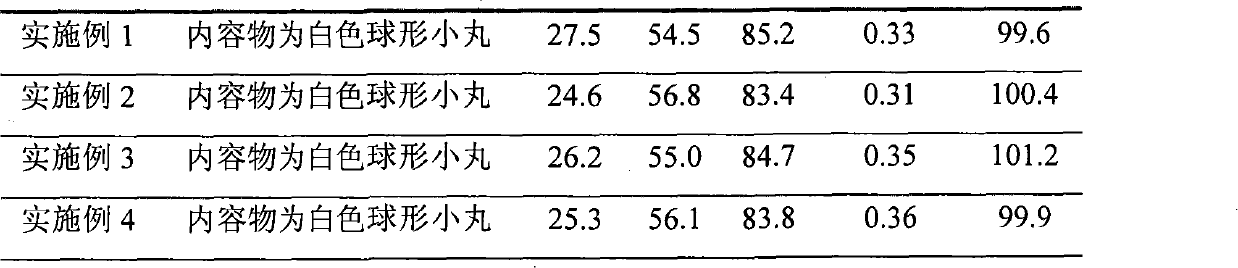
Embodiment 1
[0046] Embodiment 1 Diclofenac Potassium Sustained Release Capsules
[0047] Diclofenac Potassium 100mg
[0048] Blank pellet core 300mg
[0049] Starch 33mg
[0050] Ethylcellulose 96mg
[0051] Macrogol 6000 8mg
[0053] Titanium Dioxide 3mg
[0054] The preparation steps are:
[0055] (1) Add 300g of blank pellet cores to the granulator, spray an appropriate amount of ethanol for wetting, add a mixture of 100g of diclofenac potassium and 33g of starch, and process for 30 minutes to make drug-containing pellets with smooth surfaces;
[0056] (2) Dissolve 96g of ethylcellulose and 8g of polyethylene glycol 6000, 15g of talcum powder, and 3g of titanium dioxide with 95% ethanol solution to make a slow-release coating solution;
[0057] (3) Evenly spray the prepared sustained-release coating solution on the surface of the prepared drug-containing pellets, and then place the obtained sustained-release pellets in a vacuum oven at 70°C for 2 hours ...
Embodiment 2
[0059] Embodiment 2 Diclofenac Potassium Sustained Release Capsules
[0060] Diclofenac Potassium 100mg
[0061] Blank pellet core 300mg
[0062] Hypromellose 10mg
[0064] Acrylic resin 120mg
[0065] Glycerin 13mg
[0066] Micronized silica gel 15mg
[0067] Titanium Dioxide 3mg
[0068] The preparation steps are:
[0069] (1) Dissolve 100g diclofenac potassium, 10g hypromellose, and 15g talcum powder with 70% ethanol solution to make a coating suspension;
[0070] (2) Add 300g of blank pellet cores to the granulator, spray the prepared coating suspension on the surface of the blank pellet cores, and then vacuum-dry at 45°C to obtain drug-containing pellets;
[0071] (3) Dissolve 120g of acrylic resin and 13g of glycerin, 15g of micropowdered silica gel, and 3g of titanium dioxide with 40-80% ethanol solution to make a slow-release coating solution;
[0072] (4) Evenly spray the prepared sustained-release coating solution on the surface of ...
Embodiment 3
[0074] Example 3 Diclofenac Potassium Sustained Release Capsules
[0075] Diclofenac Potassium 200mg
[0076] Blank pellet core 300mg
[0077] Lactose 45mg
[0078] Microcrystalline Cellulose 37mg
[0079] Cellulose acetate 132mg
[0080] Povidone 21mg
[0081] Triethyl Citrate 22mg
[0082] Talc 34mg
[0083] The preparation steps are:
[0084] (1) Add 300g of blank ball cores to the granulator, spray an appropriate amount of 80% ethanol solution for wetting, add a mixture of 200g of diclofenac potassium, 45g of lactose, and 37g of microcrystalline cellulose, and place for 90 minutes to make a drug-containing pellet with a smooth surface. pellets;
[0085] (2) Dissolve 153g cellulose acetate, 22g triethyl citrate, 21g povidone, 34g talcum powder with 60%~95% ethanol solution to make slow-release coating liquid;
[0086] (3) Evenly spray the prepared sustained-release coating solution on the surface of the prepared drug-containing pellets, and then place the obtained ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com