Double layer laminating sustained release tablet of metronidazole and its preparation method
A technology of metronidazole and sustained-release tablets, which is applied in the direction of drug combination, pharmaceutical formula, drug delivery, etc., and can solve problems such as frequent taking and large gastrointestinal reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1. Preparation of metronidazole double-layer laminated sustained-release tablets
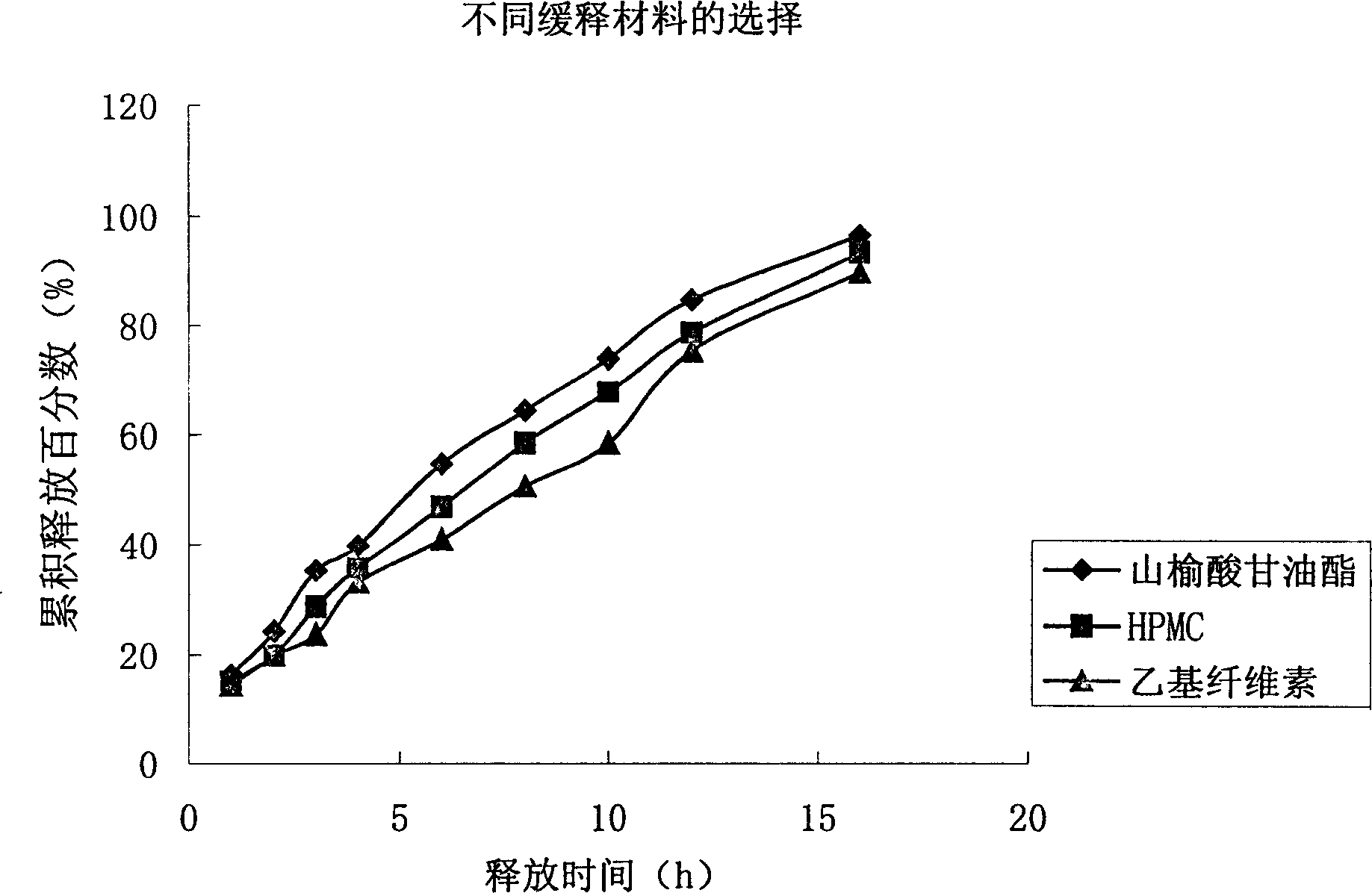
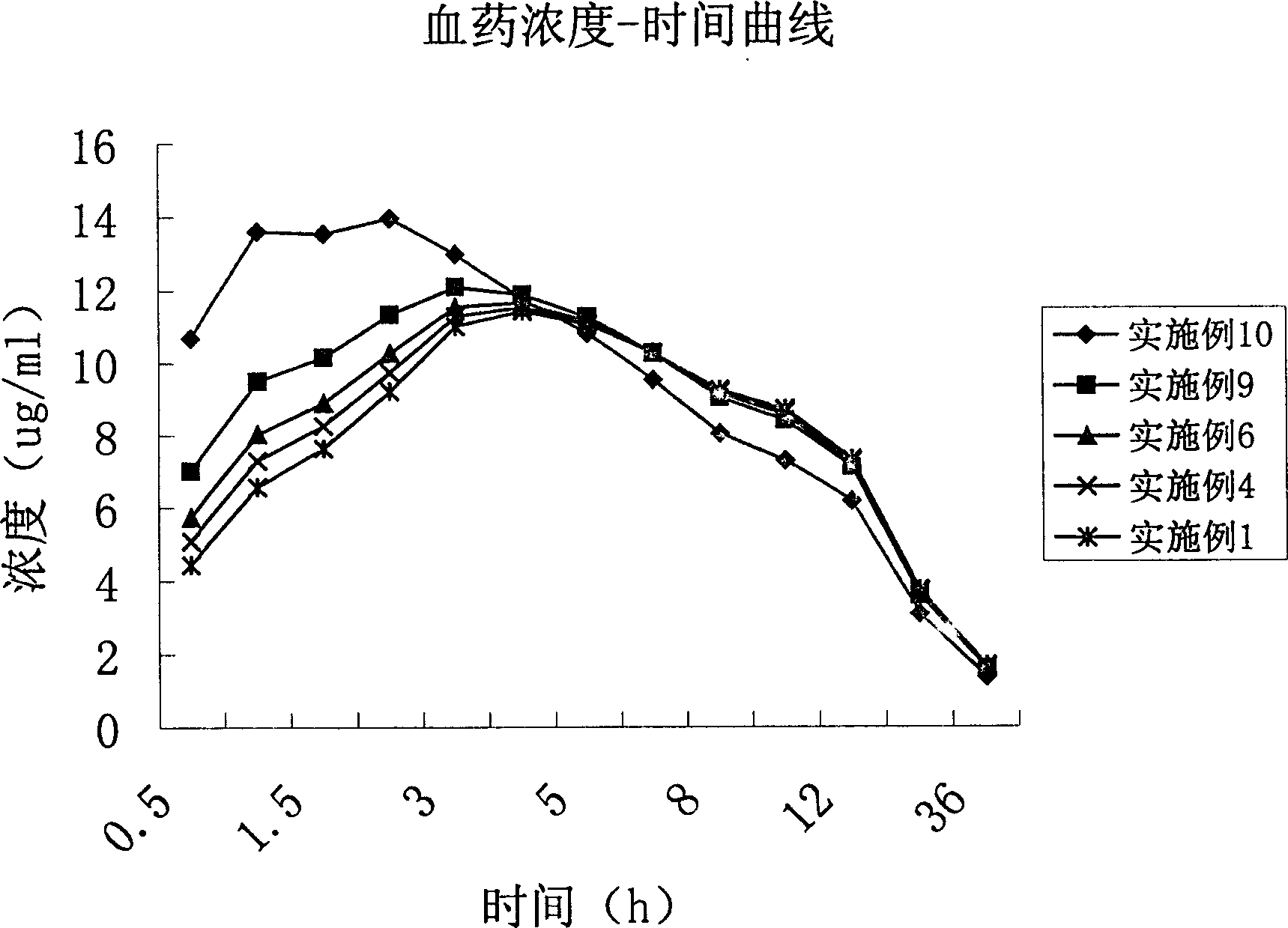
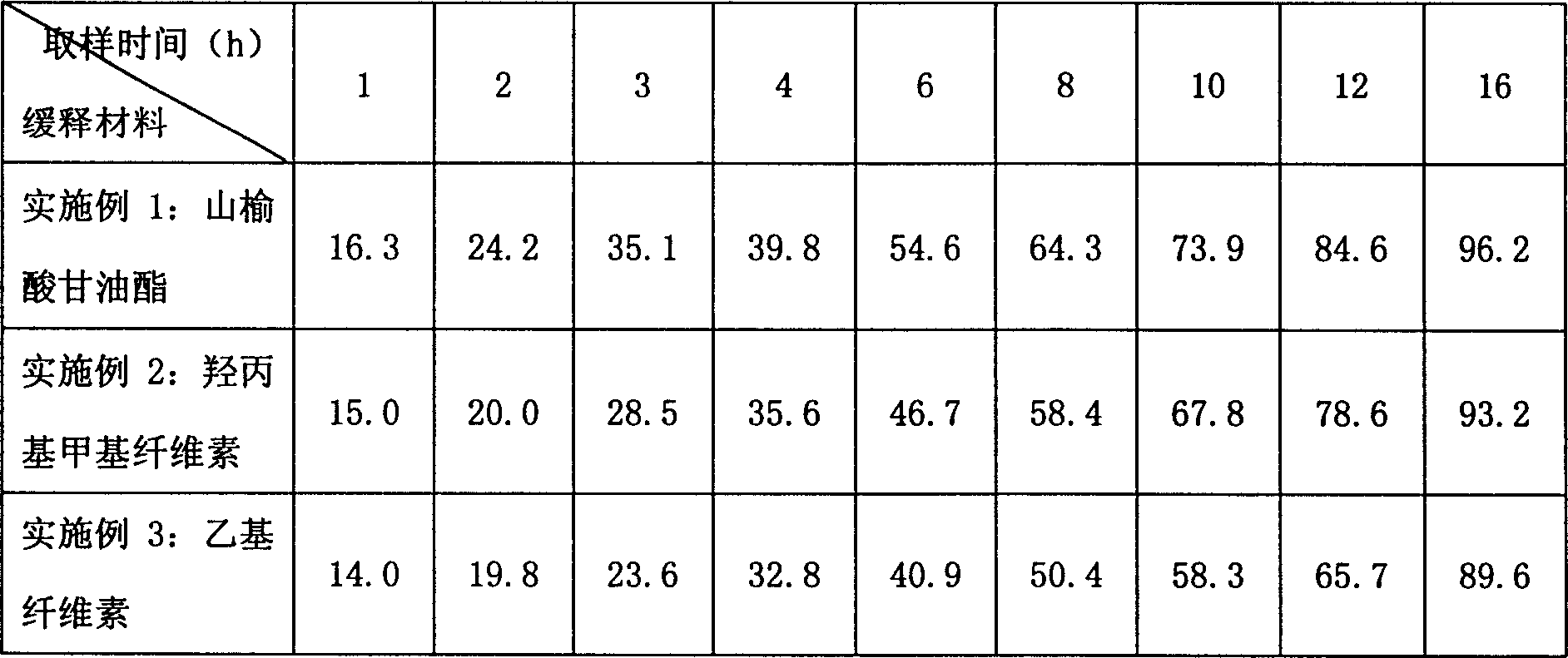
[0032] First, put the original and auxiliary materials under the condition of 60 ℃ and dry for 4 hours, and set aside; then, prepare the materials for the slow-release layer: take 550 g of metronidazole, 100 g of glyceryl behenate, and 50 g of polyvinylpyrrolidone (PVP) K30 in equal amounts Mix evenly by incremental method, pass through a 60-mesh sieve, add 20% dilute ethanol solution to make a soft material, pass through a 20-mesh sieve to make wet granules, dry at 50°C-60°C for 3-4 hours, pass through a 18-mesh sieve for granulation, and weigh. Get the sustained-release layer granules; the quick-release layer is prepared: get metronidazole 200g, microcrystalline cellulose 60g, polyvinylpyrrolidone 20g, polyvinylpyrrolidone (PVP) K30 8g and mix uniformly by the method of equal increments, cross 60 orders Sieve; add 20% dilute ethanol solution to make soft materials, pass through ...
Embodiment 2
[0033] Example 2. Preparation of metronidazole double-layer laminated sustained-release tablets
[0034] First, put the original and auxiliary materials at 60°C and dry them for 4 hours, and set them aside; then, prepare the materials for the slow-release layer: take 550 g of metronidazole, 100 g of hydroxypropyl methylcellulose (HPMC), and 100 g of polyvinylpyrrolidone (PVP) K30 15g is mixed evenly according to the method of equal increase, passed through a 60-mesh sieve, added 20% dilute ethanol solution to make soft materials, passed through a 20-mesh sieve to make wet granules, dried at 50°C-60°C for 3-4 hours, passed through a 18-mesh sieve granules, weighed to obtain the sustained-release layer granules; quick-release layer preparations: get metronidazole 200g, microcrystalline cellulose 60g, crospovidone 20g, polyvinylpyrrolidone (PVP) K30 8g and mix by equal increment method Evenly, pass through a 60-mesh sieve; add 20% dilute ethanol solution to make a soft material, ...
Embodiment 3
[0036] First, dry the raw materials and auxiliary materials at 60°C for 4 hours, and set aside; then, prepare the slow-release layer: take 550 g of metronidazole, 70 g of ethyl cellulose, and 15 g of polyvinylpyrrolidone (PVP) K30 in equal increments Mix evenly by 60-mesh sieve to get mixed powder ①; quick-release layer preparation: take metronidazole 200g, microcrystalline cellulose 60g, crospovidone 20g, ethyl cellulose 12g and mix evenly by equal increment method , through a 60-mesh sieve to obtain mixed powder ②; dry granulate mixed powder ① and mixed powder ② respectively; tabletting: add 1% micropowder silica gel to the granules of the slow-release layer and the quick-release layer respectively, mix well, and granulate the slow-release layer. Release layer and immediate release layer granules are respectively placed in two hoppers of a double-layer tablet press, and pressed into 1000 tablets on the double-layer tablet press, each containing 750 mg of metronidazole.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com