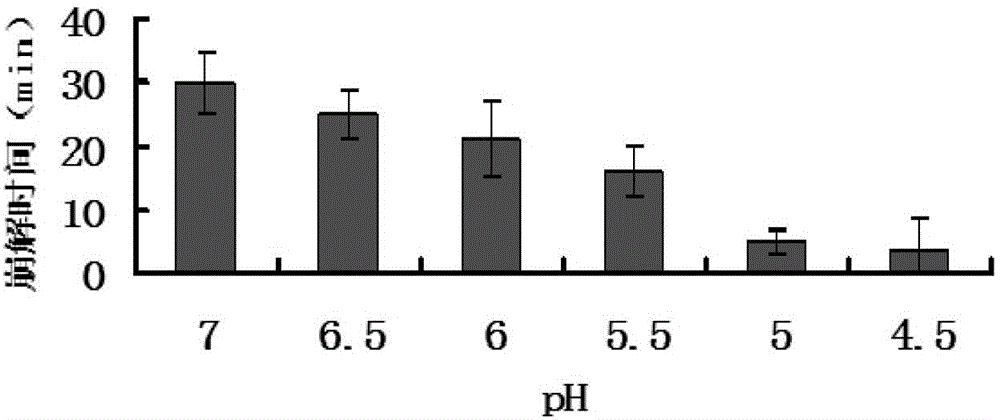
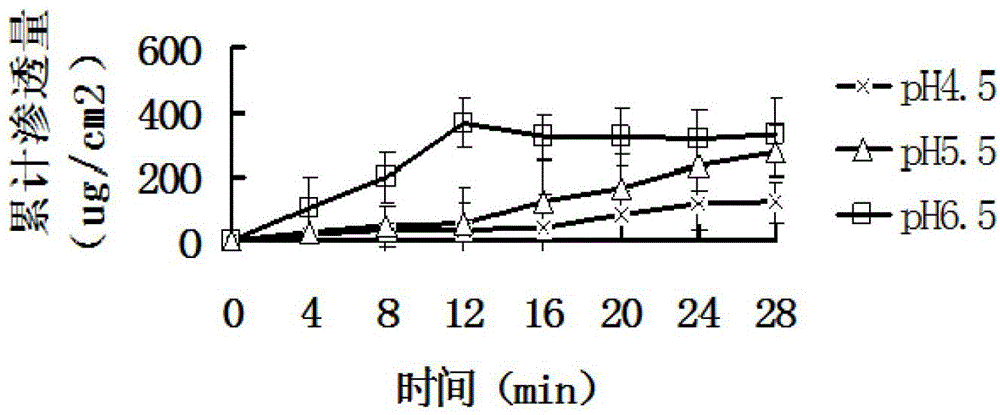
Fentanyl double-layer buccal tablet and preparation method thereof
A buccal tablet and fentanyl citrate technology, applied in the field of fentanyl double-layered buccal tablet and its preparation, can solve the problems of short analgesic effect, can not fully meet the needs of analgesia and the like, and achieve convenient use Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Preparation of Fentanyl Bilayer Buccal Tablets
[0043] Outer Immediate Release Layer Formula:
[0044] Fentanyl Citrate (1.5mg / tab), Mannitol (90.5mg / tab), Sodium Bicarbonate (42mg / tab), Sodium Carbonate (20mg / tab), Citric Acid (30mg / tab), Ac- Di-Sol Eddie Su Croscarmellose sodium (CMC-Na) (6mg / tab), magnesium stearate (4mg / tab), polyethylene glycol PEG4000 (5mg / tab), red iron oxide (1mg / tab), stevia glycosides (5mg / tab), total 200mg / tab.
[0045] Inner sustained-release layer formula: fentanyl citrate (3.0mg / tab), mannitol (90mg / tab), hypromellose HPMC K4M (42mg / tab), HPMC K15M (20mg / tab), lactose (30mg / tab), magnesium stearate (4mg / tab), red iron oxide (1mg / tab), stevioside (5mg / tab), total 200mg / tab.
[0046] Among the above components, red iron oxide can be replaced by carotene or limonin; stevioside can be replaced by asparagine phenylalanine methyl ester, apple essence, orange essence or banana essence.
[0047] Each component is pulverized and...
Embodiment 2
[0048] Example 2: Preparation of Fentanyl Bilayer Buccal Tablets
[0049] Outer Immediate Release Layer Formula:
[0050] Fentanyl citrate (1.5mg / tab), mannitol (45mg / tab), sodium bicarbonate (21mg / tab), citric acid (15mg / tab), sodium carbonate (10mg / tab), starch lactose complex STARLAC (3mg / tab), magnesium stearate (2mg / tab), carbopol 934NF (3mg / tab), red iron oxide (0.5mg / tab), stevioside (2mg / tab), total 100mg / tab.
[0051] Inner slow-release layer formula:
[0052] Fentanyl Citrate (3.0mg / tab), Mannitol (45mg / tab), Hypromellose HPMC K15M (20mg / tab), HPMC K4M (11mg / tab), Lactose (15mg / tab), Hard Magnesium fatty acid (2mg / tab), red iron oxide (0.5mg / tab), stevioside (2mg / tab), total 100mg / tab.
[0053] Using dry powder or wet tableting, pre-compress the slow-release granules first, and adjust the tablet weight and pre-compression pressure to 1-10kg / cm 2 , and then adding the immediate-release layer granules for compression to obtain a double-layer oral buccal tablet ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com