Florfenicol premixing agent and preparation method thereof
A technology of florfenicol and premix, applied in the direction of non-active ingredients of polymer compounds, powder delivery, antibacterial drugs, etc., can solve the problems of low oral bioavailability, slow dissolution rate in vitro, unfavorable generalization, etc. Achieve the effect of meeting the needs of rapid growth of animals, high biological activity and utilization, and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
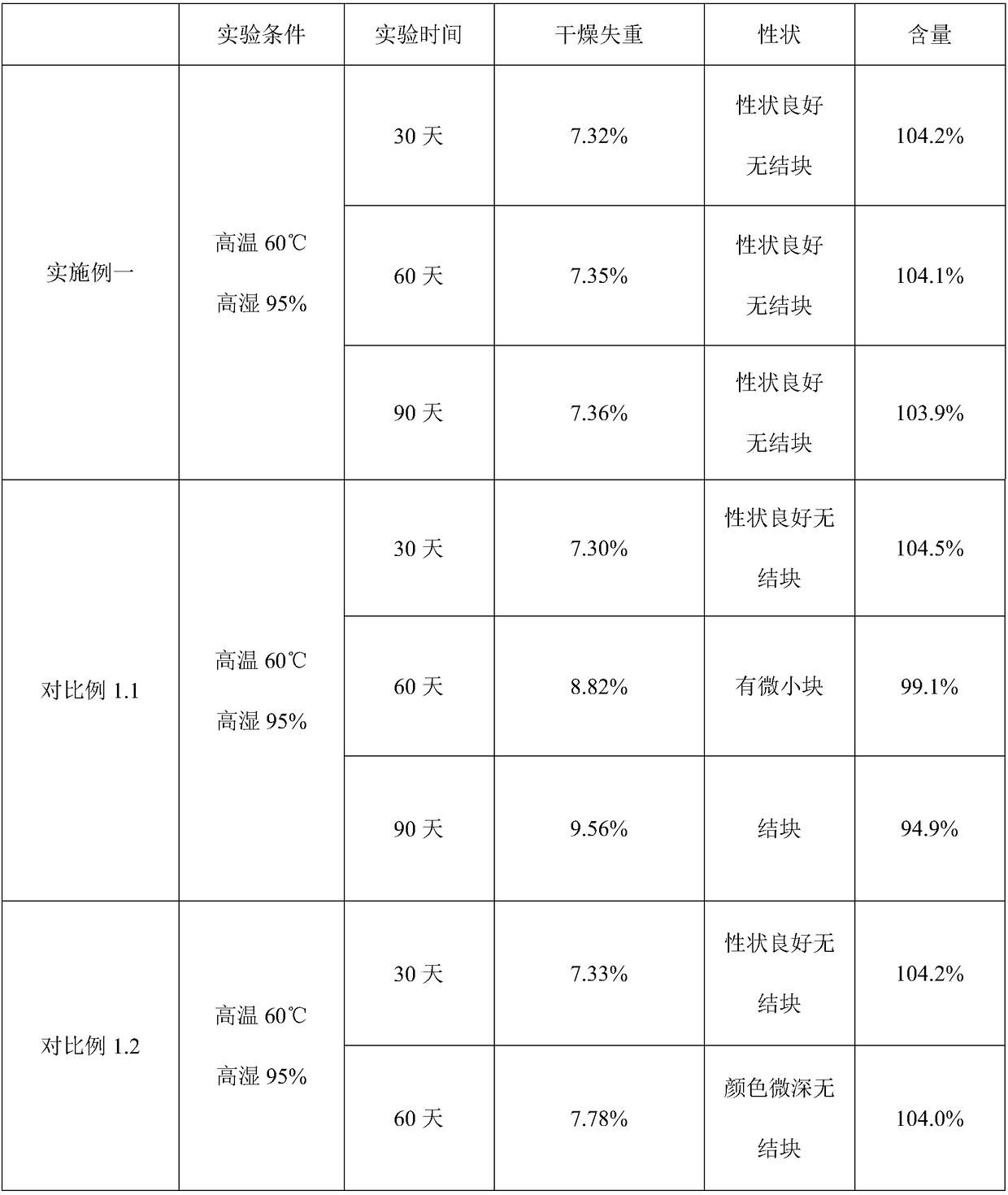
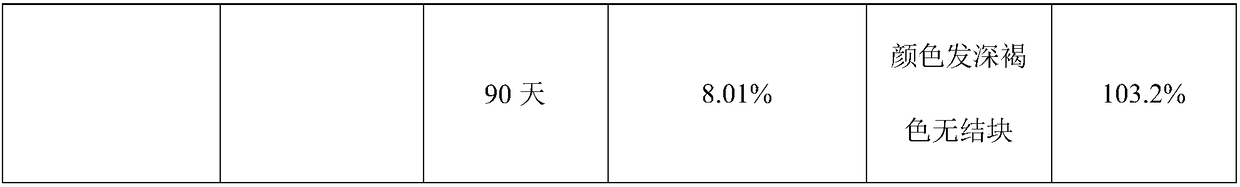
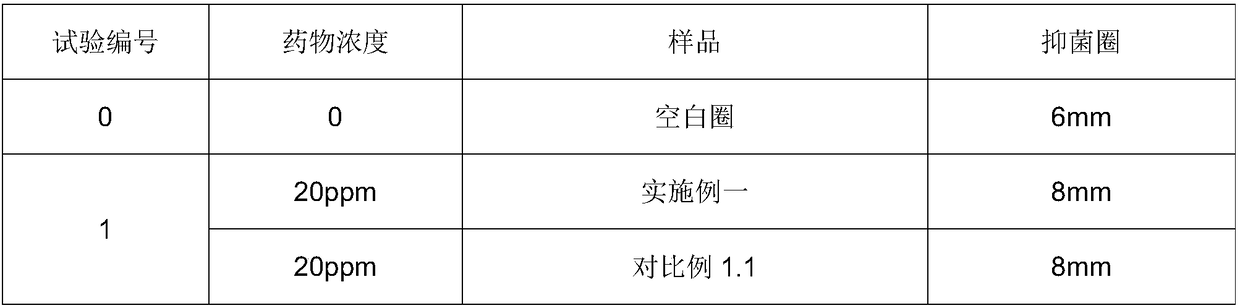
Examples
Embodiment 1
[0026] This embodiment relates to Florfenicol premix and its preparation.
Embodiment 11
[0028] A kind of Florfenicol premix, its raw material component (by weight) comprises:
[0029] Florfenicol crude drug 2 parts,
[0030] Active auxiliary material: 5 parts of disodium salt of ethylenediaminetetraacetic acid,
[0031] Carrier: 5 parts of water-soluble epichlorohydrin cross-linked α-cyclodextrin,
[0032] Dissolution agent: polyethylene glycol 4000 10 parts,
[0033] Stabilizer: 3 parts of micropowder silica gel,
[0034] Filling agent: add montmorillonite to 100 parts;
[0035] Take the above-mentioned raw material components, prepare Florfenicol premix according to the following steps:
[0036] a. The florfenicol bulk drug and active excipients were reacted by solid-phase grinding method, and ground for 15 minutes at normal temperature to obtain a mixture A with a grinding particle size of 80 mesh sieve;
[0037] b. The mixture A is subjected to a grinding process with a carrier and an eluting agent, and ground at room temperature for 20 minutes to obtain...
Embodiment 12
[0040] A kind of Florfenicol premix, its raw material component (by weight) comprises:
[0041] Florfenicol crude drug 2 parts,
[0042] Active excipients: 3 parts of sodium lauryl sulfate, 3 parts of magnesium lauryl sulfate,
[0043] Carrier: 5 parts of water-soluble epichlorohydrin cross-linked β-cyclodextrin,
[0044] Dissolution agent: 5 parts of polyethylene glycol 4000, 5 parts of polyethylene glycol 6000
[0045] Stabilizer: 7 parts of light magnesium oxide,
[0046] Filler: soybean lecithin, defatted soybean powder added to 100 parts;
[0047] Take the above-mentioned raw material components, prepare Florfenicol premix according to the following steps:
[0048] a. The florfenicol raw material and active excipients were reacted by solid-phase grinding method, and ground at room temperature for 10 minutes to obtain a mixture A with a grinding particle size of 80 mesh sieve;
[0049] b. The mixture A is subjected to a grinding process with a carrier and an eluting a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com