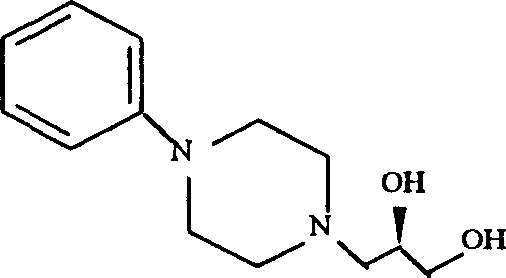
Slow release levodropropizine pharmaceutical composition
A technology of levothyroxine and sustained-release drugs, applied in the field of sustained-release drugs, can solve the problems of adverse reactions, high peak blood drug concentration, clinical application limitations and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The raw material formula is as follows:
[0020] Levodropropizine 12.4 parts
[0021] Sustained-release excipients:
[0022] Hypromellose K4M 1.5 parts
[0023] Ordinary functional accessories:
[0024] Starch 4.0 parts
[0025] Lactose 12.4 parts
[0026] Microcrystalline cellulose 16.4 parts
[0027] Magnesium stearate 0.5 part
[0028] The solvent is 2 parts of 50% ethanol in water
[0029] The hydroxypropyl methylcellulose K4M in the prescription is a hydrophilic gel skeleton, which swells to form a gel barrier when it encounters water or digestive juices, and controls the diffusion of levodropropizine to achieve the purpose of release.
[0030] The preparation method is as follows: preparing granules according to the preparation method of conventional tablets, and pressing into double-layer tablets by using a double-layer tablet press machine.
Embodiment 2
[0032] The raw material formula is as follows:
[0033] Levodropropizine 24.7 parts
[0034] Sustained-release excipients:
[0035] Hypromellose K4M 8.2 parts
[0036] Ordinary functional accessories:
[0037] Lactose 16.4 parts
[0038] Magnesium stearate 0.5 part
[0039] The solvent is 3 parts of 60% ethanol aqueous solution
[0040] The hydroxypropyl methylcellulose K4M in the prescription is a hydrophilic gel skeleton, which swells to form a gel barrier when it encounters water or digestive juices, and controls the diffusion of levodropropizine to achieve the purpose of release.
[0041] Preparation method: Prepare granules according to the conventional tablet preparation method, and press into double-layer tablets with a double-layer tablet press.
Embodiment 3
[0043] The raw material formula is as follows:
[0044] Levodropropizine 31.4 parts
[0045] Sustained-release excipients:
[0046] Hypromellose (K4M) 14.0 parts
[0047] Ordinary functional accessories:
[0048] Starch 17.4 parts
[0049] Lactose 29.6 parts
[0050] Stearic acid 2.6 parts
[0051] Magnesium stearate 0.5 parts
[0052] Talcum powder 1.5 parts
[0053] The solvent is 3 parts of 60% ethanol aqueous solution
[0054] In the prescription, hydroxypropyl methylcellulose K4M is a hydrophilic gel skeleton, and stearic acid is a bioerodible skeleton, which swells with water or digestive juice to form a gel barrier and emulsion, which controls the diffusion of levodropropizine and achieves release Purpose.
[0055] Preparation method: Prepare granules according to the conventional tablet preparation method, and press into double-layer tablets with a double-layer tablet press.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com