Pharmaceutical composition for treating or preventing bacterial and mycoplasma diseases of livestock and use thereof
A composition and bacteriological technology, applied in the direction of antibacterial drugs, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of laborious and laborious, reducing drug effects, multiple injections, etc., to achieve The effect of maintaining blood concentration, prolonging half-life, and slow release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
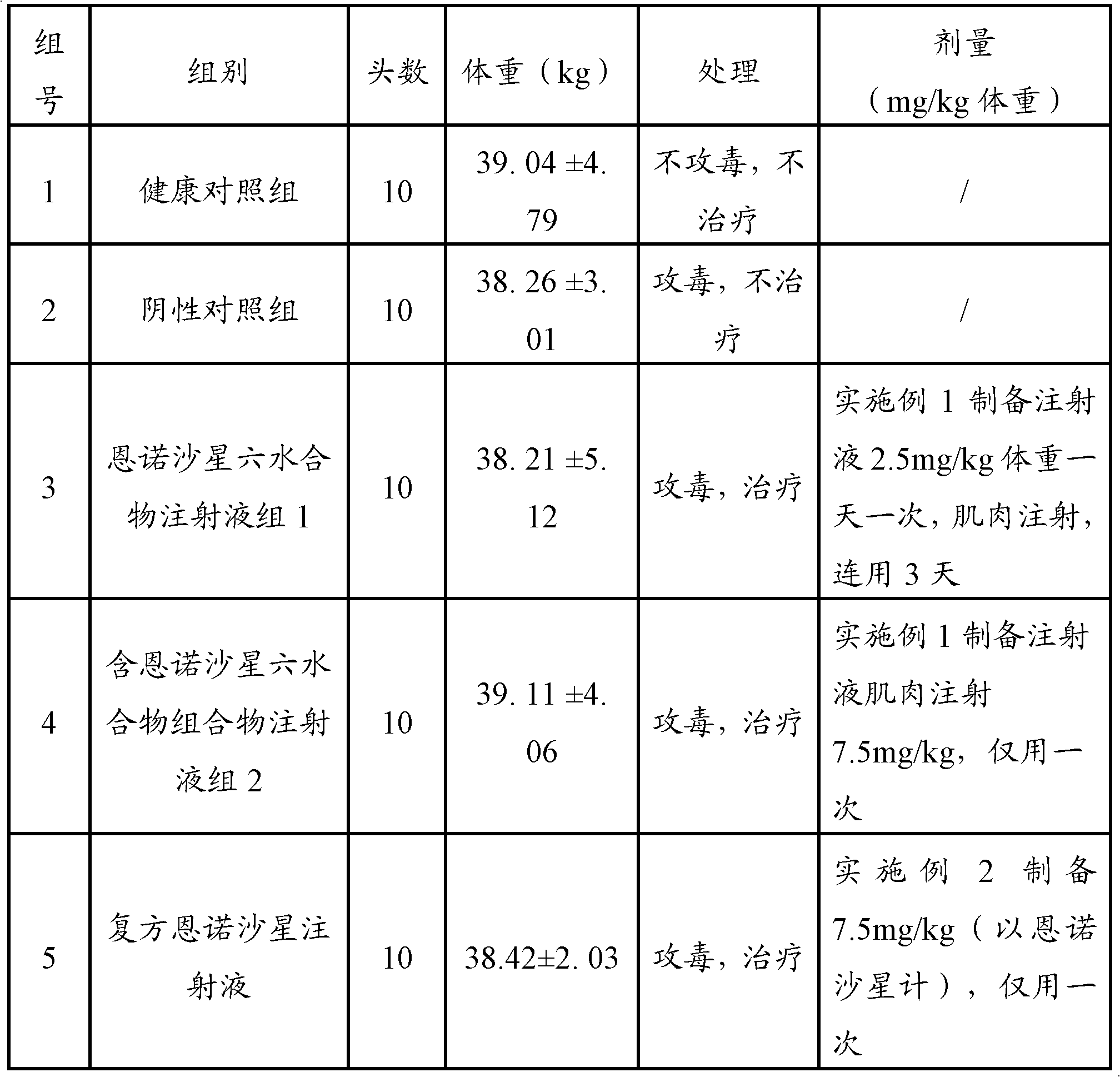
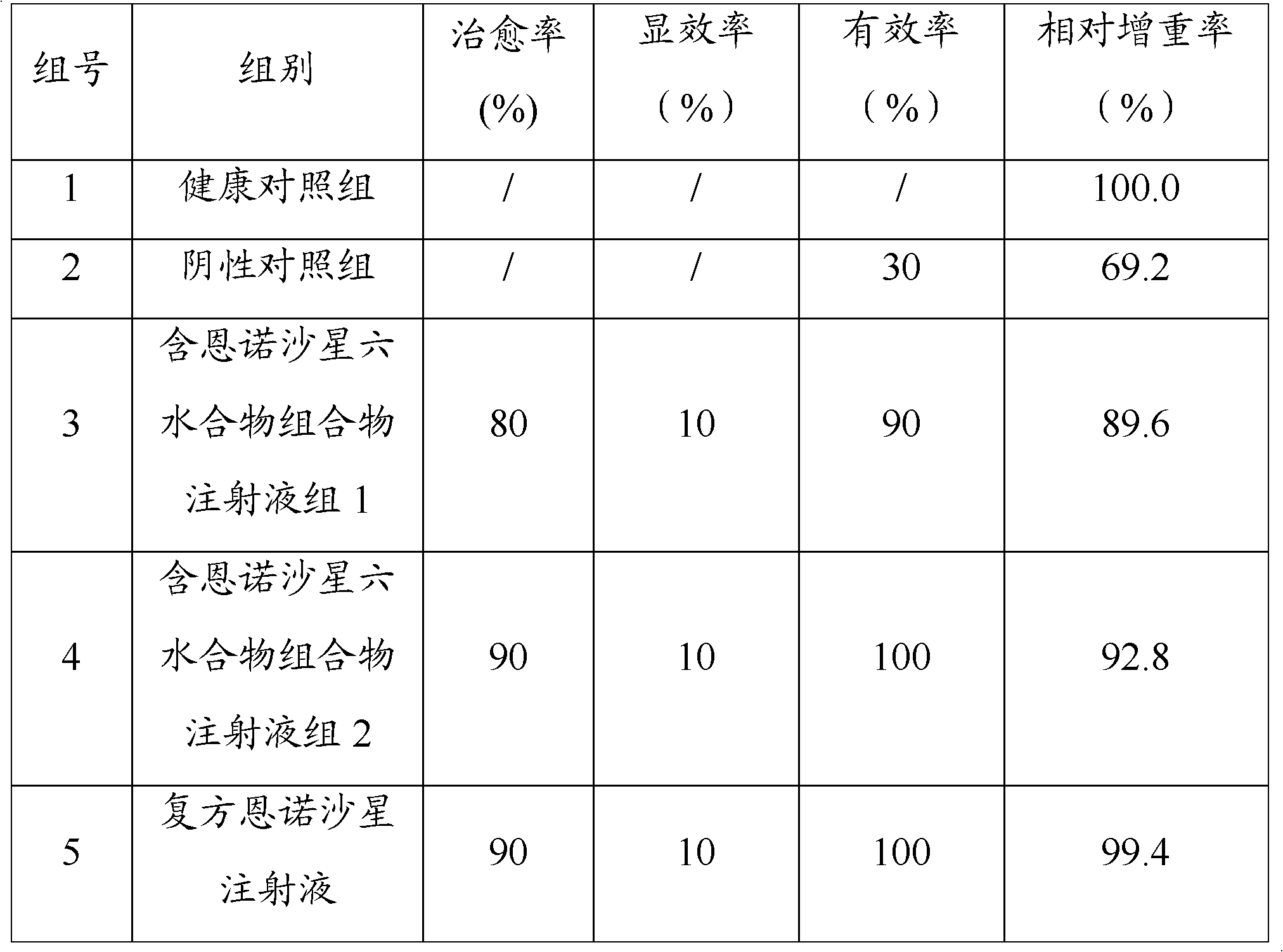
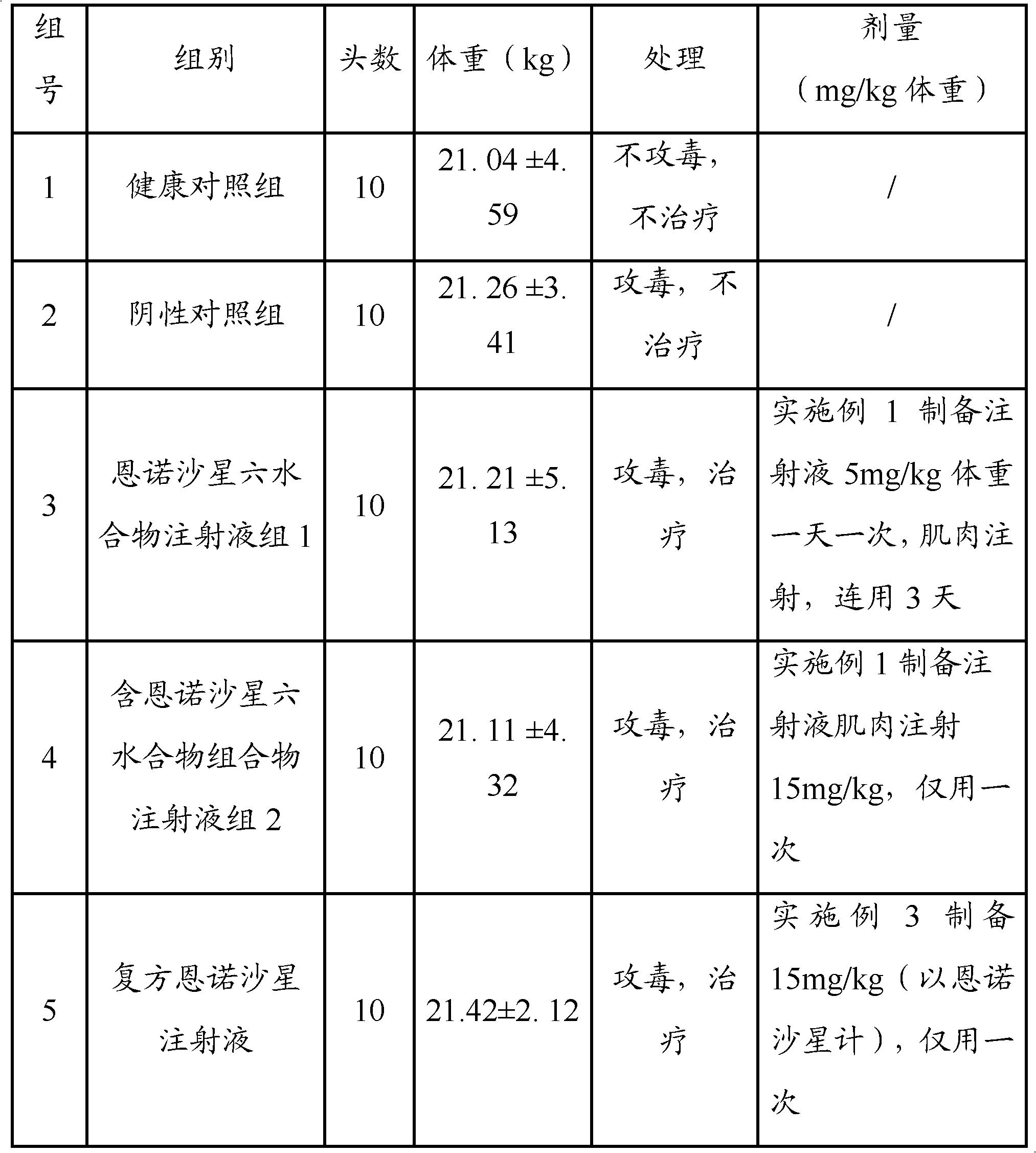
Embodiment 1
[0035] Embodiment 1, preparation containing 10% W / V enrofloxacin hexahydrate injection
[0036] 1) Preparation of 10% W / V enrofloxacin hexahydrate composition
[0037] Enrofloxacin hexahydrate (prepared according to the method of CN101933927A Example 1 is 74.0% (the raw material content is calculated as enrofloxacin, the same below)): 10% W / V (calculated as enrofloxacin)
[0038] Cosolvent: KOH (Tianjin Comio) 1.5% W / V; Arginine (Hubei Yuancheng Pharmaceutical Co., Ltd.) 10% W / V
[0039] Preservative: n-Butanol 3% W / V
[0040] Carrier: water for injection to a full dose of 1000ml
[0041] Preparation steps:
[0042] 1) Preparation of KOH solution: Weigh 15g of KOH, add 50ml of water for injection, stir to dissolve and dilute to 100ml;
[0043] 2) Mix 600ml of water for injection and 30g of n-butanol evenly, and control the temperature at 20°C-30°C during mixing;
[0044] 3) Add 135.1 g of enrofloxacin hexahydrate and stir to mix evenly;
[0045] 4) Slowly add the above K...
Embodiment 2
[0047] Embodiment 2, preparation of compound 10% W / V enrofloxacin hexahydrate injection
[0048] Enrofloxacin hexahydrate is prepared according to (according to CN101933927A embodiment 1, content is 74.0% based on enrofloxacin): 10% W / V
[0049] Butafosfos (Hangzhou Foster Pharmaceutical Co., Ltd. content 99.8%) 2.5W / V
[0050] Cosolvent: KOH 1.5% W / V Arginine 10% W / V
[0051] Preservative: n-Butanol 3% W / V
[0052] Carrier: water for injection to the full amount of 1000ml
[0053] Preparation steps:
[0054] 1) Preparation of KOH solution: Weigh 15g of KOH, add 100ml of water for injection, stir to dissolve and set the volume to 200ml;
[0055] 2) Mix 600ml of water for injection and 30g of n-butanol evenly, and control the temperature at 20°C-30°C during mixing;
[0056] 3) Add 135.1 g of enrofloxacin hexahydrate and stir to mix evenly;
[0057]4) Slowly add the above-mentioned KOH solution, then add 100g of arginine and 25g of butafosf in turn and stir; stir to dissol...
Embodiment 3
[0059] Embodiment 3, preparation of compound 15% W / V enrofloxacin hexahydrate injection
[0060] Enrofloxacin hexahydrate (prepared according to N101933927A Example 1 content is 74.0% based on enrofloxacin): 15% W / V
[0061] Butafosfos (Hangzhou First Pharmaceutical Co., Ltd. content 99.8%) 4.0% W / V
[0062] Co-solvent: KOH 3.0%W / V
[0063] Preservative: n-Butanol 3% W / V
[0064] Carrier: water for injection to the full amount of 1000ml
[0065] Preparation steps:
[0066] 1) Preparation of KOH solution: Weigh 30g of KOH, add 100ml of water for injection, stir to dissolve and set the volume to 200ml;
[0067] 2) Mix 600ml of water for injection and 30g of n-butanol evenly, and control the temperature at 20°C-30°C during mixing;
[0068] 3) Add 202.3 g of enrofloxacin hexahydrate and stir to mix evenly;
[0069] 4) Slowly add the above KOH solution, and stir butafosfos 40g to dissolve it;
[0070] 5) Dilute the volume of water for injection to 1000ml, mix well, and filte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com