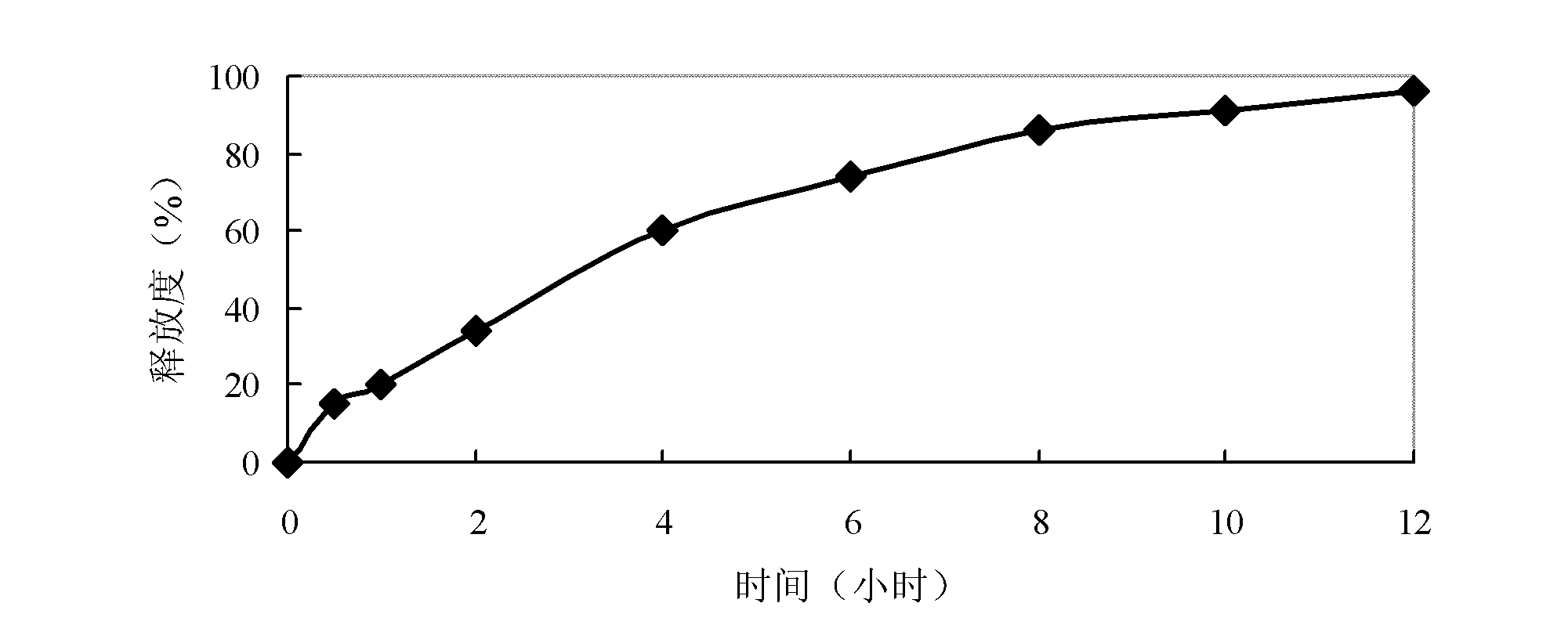
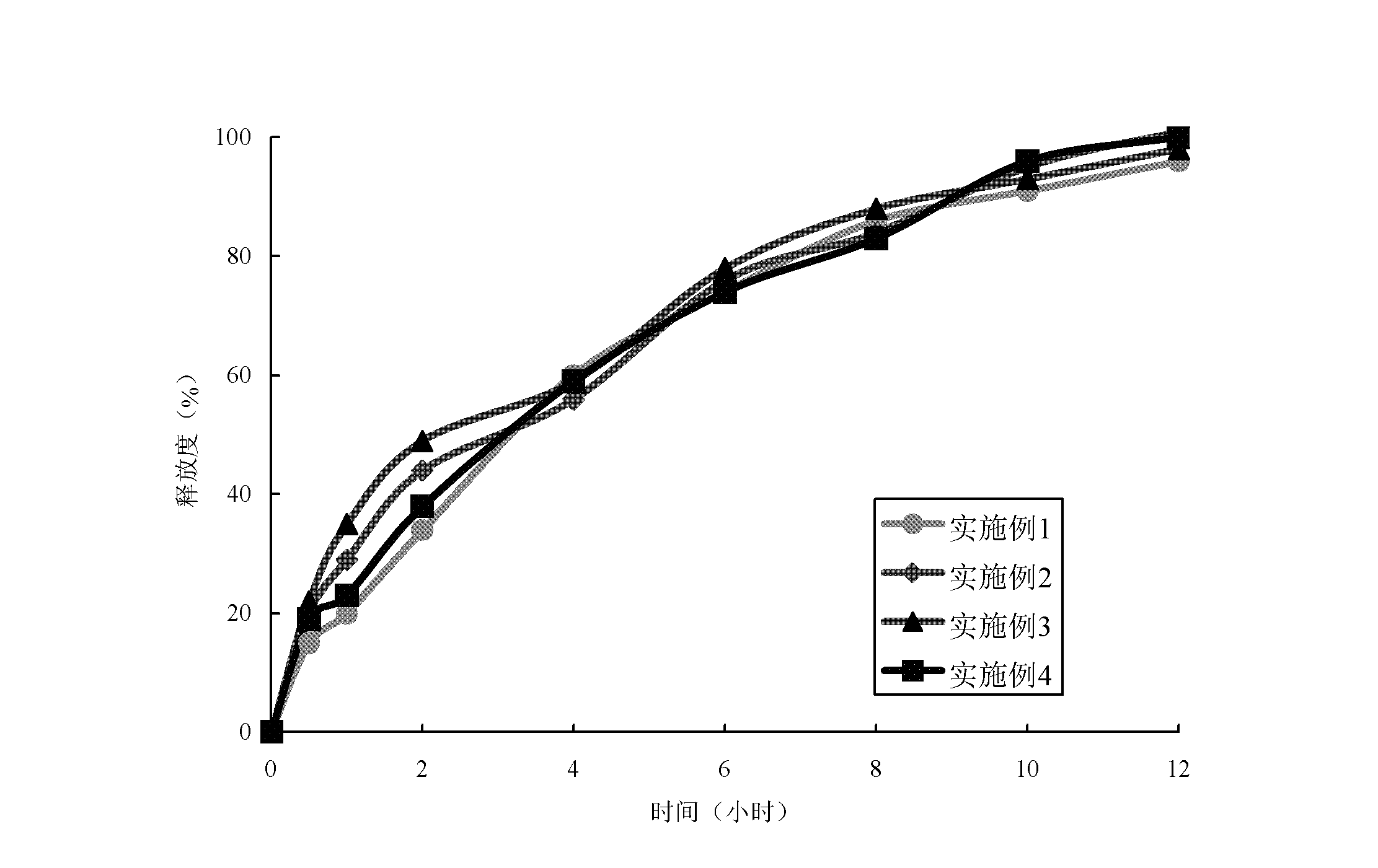Dihydroartemisinin controlled-release preparation used for treating lupus erythematosus
A technology of dihydroartemisinin and controlled-release preparations, which is applied in the field of pharmacy, can solve the problems of short half-life of dihydroartemisinin and inconvenient medication, and achieve the effects of reduced treatment cost, convenient medication and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Immediate-release pellets: 20g of dihydroartemisinin of 10-50μm, 10g of mannitol, 10g of lactose, 26g of microcrystalline cellulose, 6g of crospovidone, 0.2g of butylated hydroxytoluene, 0.1g of citric acid, hydroxypropyl 8 g of cellulose and 20 g of 95% (v / v) ethanol are used as binders to make quick-release pellets.
[0033] Controlled-release pellet core: 80g dihydroartemisinin 10-50μm, 100g sucrose, 147g starch, 60g dextrin, 136g microcrystalline cellulose, 27g povidone, 2g butylated hydroxytoluene, 1g citric acid, hypromellose Cellulose 14g, 70% (v / v) ethanol 350g are wetting agent, make controlled-release ball core.
[0034] Sublayer coated pellets: 540 g of controlled-release ball cores, coated with 5.4 g of zein protein and 270 g of 95% (v / v) ethanol, with a weight gain of about 1%.
[0035] Coated pellets 1: 135g of coated bottom pellets, coated with ethyl cellulose 2g, hypromellose 0.4g, polyethylene glycol-400 0.3g, 95% (v / v) ethanol 70g mixed solution coati...
Embodiment 2
[0040] Dihydroartemisinin solid dispersion: 10 g of povidone is dissolved in 100 g of 95% (v / v) ethanol, 20 g of dihydroartemisinin is added to dissolve it, and the solution is adsorbed on 30 g of crospovidone, Evaporate the solvent to make fine particles.
[0041] Immediate-release pellets: 60g of dihydroartemisinin solid dispersion, 5g of croscarmellose sodium, 5g of lactose, 10g of microcrystalline cellulose, 0.2g of butylated hydroxytoluene, 0.1g of citric acid, and 2.1g of povidone 1. Distilled water 30g is a wetting agent, made into quick-release pellets.
[0042] Controlled-release pill core: micronized dihydroartemisinin 80g, starch 117g, polyethylene glycol-6000 150g, hypromellose 80g, microcrystalline cellulose 110g, butylated hydroxytoluene 2g, citric acid 1g, hypromellose Cellulose 20g, 70% ethanol solution 500g are wetting agent, make controlled-release ball core.
[0043] Sublayer coat pellets: 540 g of controlled-release ball cores, coated with a mixture of No...
Embodiment 3
[0049] Immediate-release pellets: 20g of dihydroartemisinin of 10-50μm, 35g of microcrystalline cellulose, 4g of crospovidone, 0.3g of butylated hydroxytoluene, 0.2g of citric acid, 3g of povidone, 30g of 95% ethanol As a binder, it is made into quick-release pellets.
[0050] Controlled-release pellet core: 60g of dihydroartemisinin with a diameter of 10-50 μm, 120g of sucrose, 300g of microcrystalline cellulose, 27g of povidone, 3g of butylated hydroxytoluene, 30g of hydroxypropyl cellulose and 300g of distilled water as wetting agents. Formed into a controlled-release pellet core.
[0051] Coated pellet 1: 67.5g of controlled-release pellet core, wrapped with 1g of hypromellose, povidone 1, 0.5g of polyethylene glycol-400, 0.5g of glycerin, and 150g of 70% (v / v) ethanol clothing, gaining about 2% in weight.
[0052] Coated pellet 2: 135g of controlled-release pellet core, coated with No. IV acrylic resin 4g, povidone 1g, polyethylene glycol-400 0.2g, glycerin 0.2g, 95% (v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com