Compound olanzapine subcutaneous implantable sustained-release preparation
A compound olanzapine and olanzapine technology, applied in the field of antipsychotic medical drugs, can solve the problems of insurmountable olanzapine hygrosensitivity, poor compliance with maintenance treatment, low bioavailability and the like, and achieve stable maintenance treatment effect, Mild toxic and side effects in humans and reliable drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
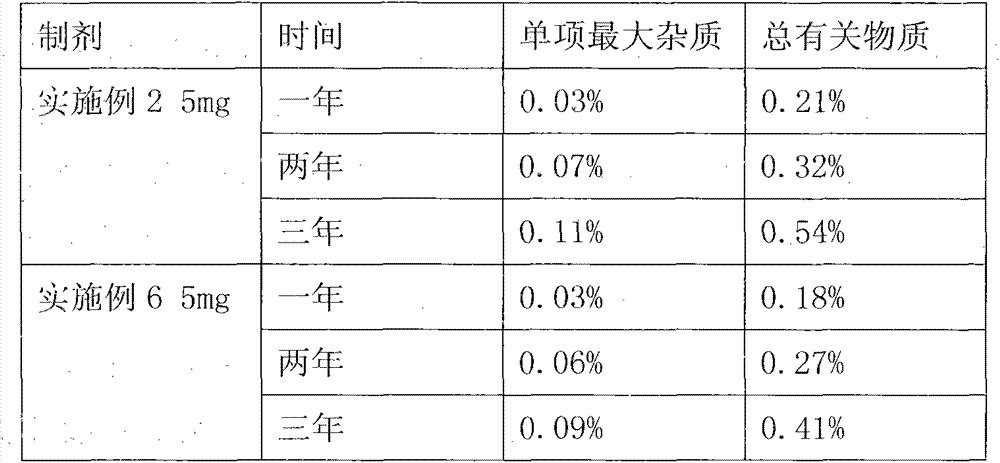
Embodiment 1
[0027] Take 150mg olanzapine granules plus 1.5ul propionic acid as a controlled release agent, prepare it as a preparation, the steps are as follows:
[0028] 1) Preparation of raw materials: take the required medical silicone tube, olanzapine plain drug, controlled release agent, and medical adhesive for quality confirmation and disinfection;
[0029] 2) Compound drug preparation: put olanzapine and propionic acid into a container, add 60% ethanol, stir fully under normal temperature and pressure to make a uniform suspension, and make ethanol under normal temperature and pressure conditions Completely volatilize to obtain the agglomerate of compound olanzapine, and crush the agglomerate of compound olanzapine into 400 mesh powder under aseptic and normal temperature conditions for subsequent use;
[0030] 3) Compound drug filling: according to the measurement requirements, the powder is filled into medical silicone tubes at room temperature and pressure under the condition of...
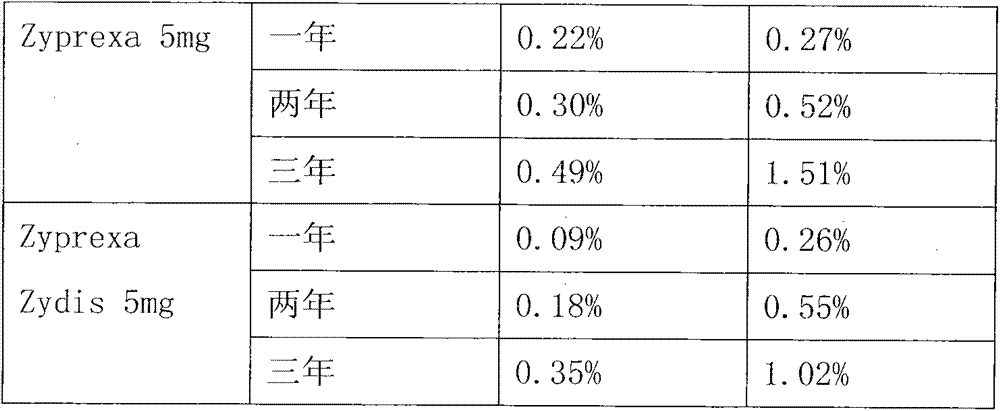
Embodiment 2
[0035] Take 150mg olanzapine granules and add 0.5ul propionic acid as a controlled release agent, prepare it as a preparation, the preparation steps are the same as in Example 1; wherein the medical adhesive used is medical silica gel, the organic solvent used is 70% ethanol, and the filling specification 160mg per tube.
Embodiment 3
[0037] Take 150mg olanzapine granules and add 0.25ul propionic acid as a controlled release agent, prepare it as a preparation, the preparation steps are the same as in Example 1. The medical adhesive used is medical silica gel, the organic solvent used is 90% ethanol, and the filling specification 150mg per tube.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com