Vonoprazan oral quick-dissolving film agent and method for preparing same
An oral instant film and plasticizer technology, applied in the field of medicine, can solve problems such as difficulty in products, and achieve the effects of improving compliance, solving long disintegration time, and being convenient to carry.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
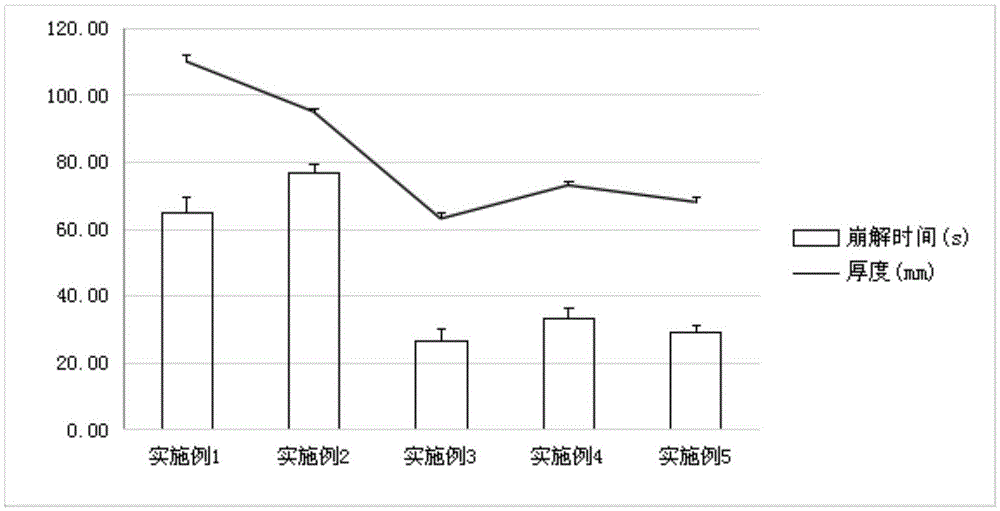
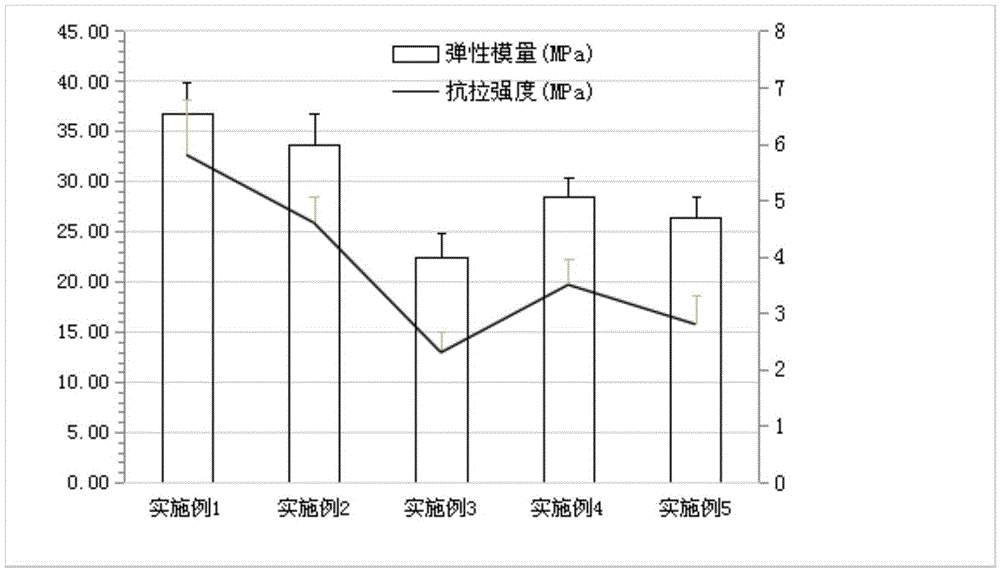
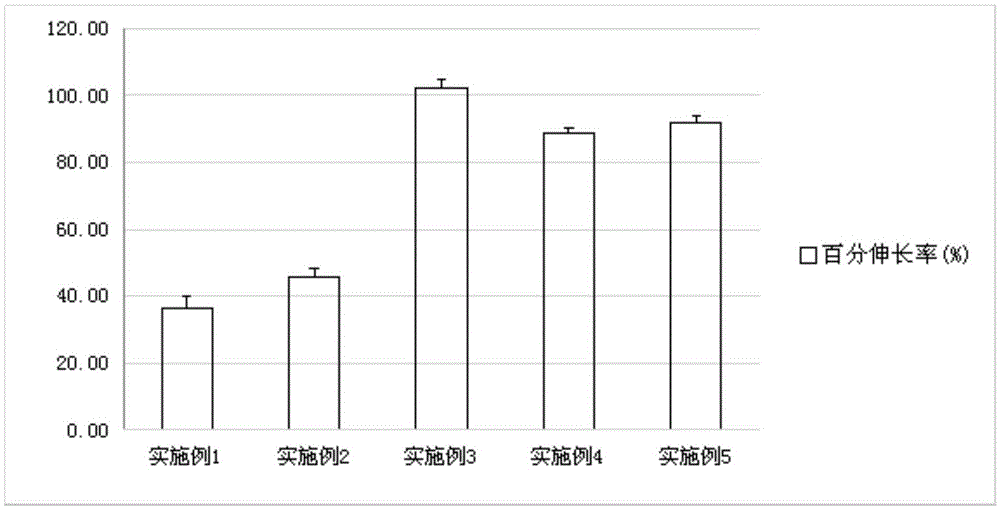
Examples
Embodiment 1
[0041] Example 1: Preparation of oral instant blank film.
[0042]
[0043] First add the above-mentioned amount of hypromellose, maltodextrin, and hyaluronic acid into pure water under stirring, stir and dissolve to obtain a gel solution, then add glycerin, and stir evenly. Allow the prepared solution to stand or sonicate to remove air bubbles. Spread the solution evenly on 15×15cm 2 Stainless steel plate, 40-60 ℃ blast heating and drying for 2 hours. Remove the film and cut it to get the film agent, which is white in color, easy to remove the film, with average toughness and brittleness.
Embodiment 2
[0044] Example 2: Preparation of oral instant blank film.
[0045]
[0046] First add the above-mentioned amount of hypromellose, maltodextrin, and hyaluronic acid into pure water under stirring, stir and dissolve to obtain a gel solution, then add glycerin, and stir evenly. Allow the prepared solution to stand or sonicate to remove air bubbles. Spread the solution evenly on 15×15cm 2 Stainless steel plate, 40-60 ℃ blast heating and drying for 2 hours. Remove the film and cut it to get the film agent, which is transparent, tough and has good release performance.
Embodiment 3
[0047] Example 3: Preparation of oral instant blank film.
[0048]
[0049] First add the above-mentioned amount of hypromellose, maltodextrin, and hyaluronic acid into purified water under stirring, stir and dissolve to obtain a gel solution, then add glycerin, and stir evenly. Allow the prepared solution to stand or sonicate to remove air bubbles. Spread the solution evenly on 15×15cm 2 The stainless steel plate was heated and dried at 40-60°C for 2 hours. Remove the film and cut it to get the film agent. The film is transparent, has good release performance and is flexible.
PUM
| Property | Measurement | Unit |
|---|---|---|
| elastic modulus | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com