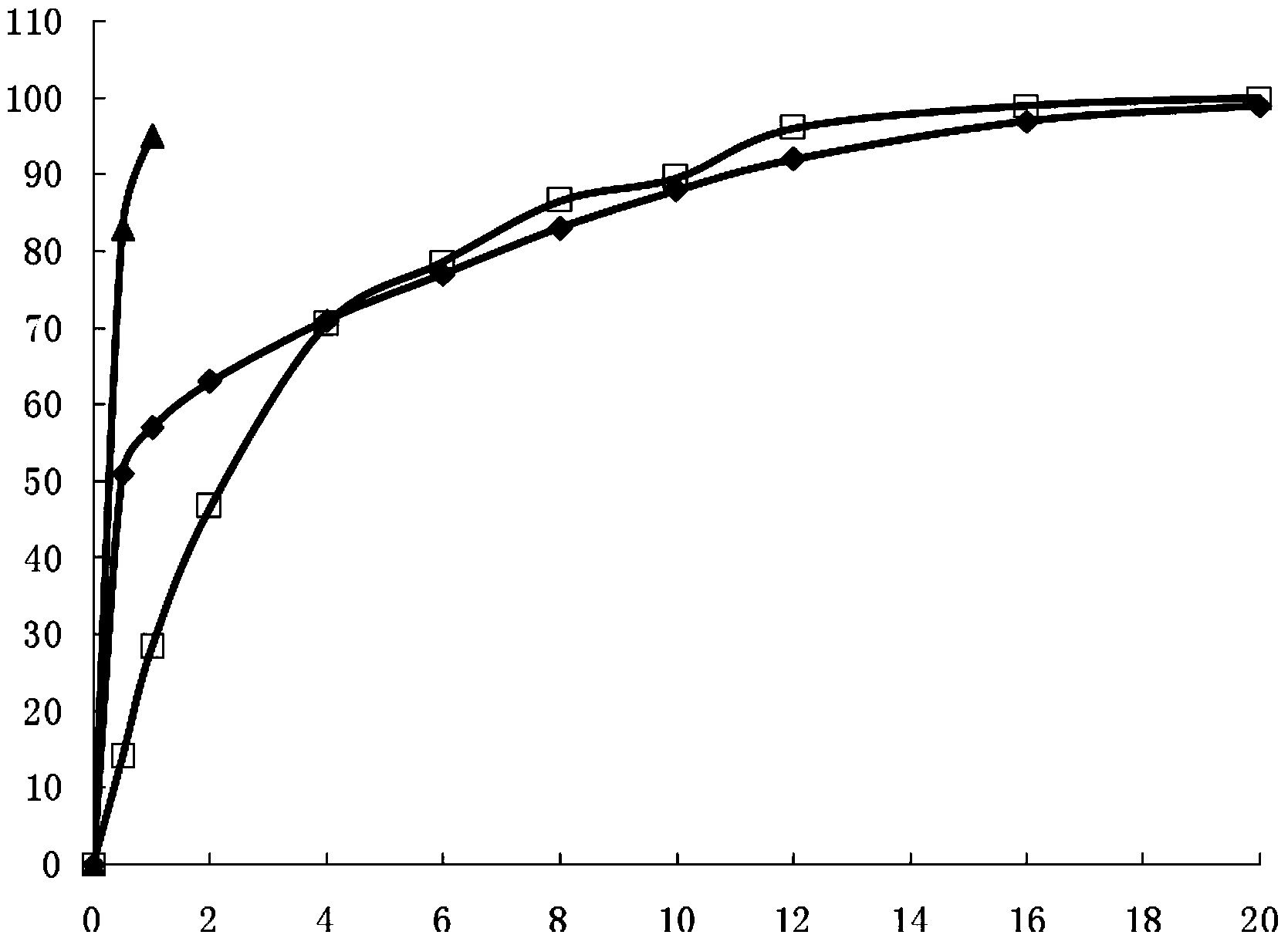Ibuprofen preparation and preparation method thereof
A preparation and diluent technology, applied in anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc., can solve the problems of no sudden release effect, affect the therapeutic effect, fast release speed, etc., achieve great clinical application value, reduce The number of doses, the effect of solving the slow onset
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061]
[0062]
[0063] Dissolve 30 grams of hypromellose in 345 grams of purified water to make an aqueous solution, mix 600 grams of ibuprofen and 300 grams of microcrystalline cellulose evenly, and use an aqueous solution of 8% hypromellose to rapidly wet granulate The wet material is prepared in the SHK-4B machine, and the wet material is made into drug-containing pellets by using the extrusion spheronizer of Chongqing Yingge, and the drug-containing pellets are dried to obtain immediate-release pellets. The received immediate-release pellets were divided into three parts. 90 grams of acrylic resin and 40 grams of talcum powder, 6 grams of triethyl citrate are made into the coating solution of 20% solid content, and two parts of micropills in the micropills collected are carried out using Gladt GPCG-2 fluidized bed Sustained-release coating to prepare sustained-release coated pellets.
[0064] Mix the sustained-release coated pellets with another part of the immedi...
Embodiment 2
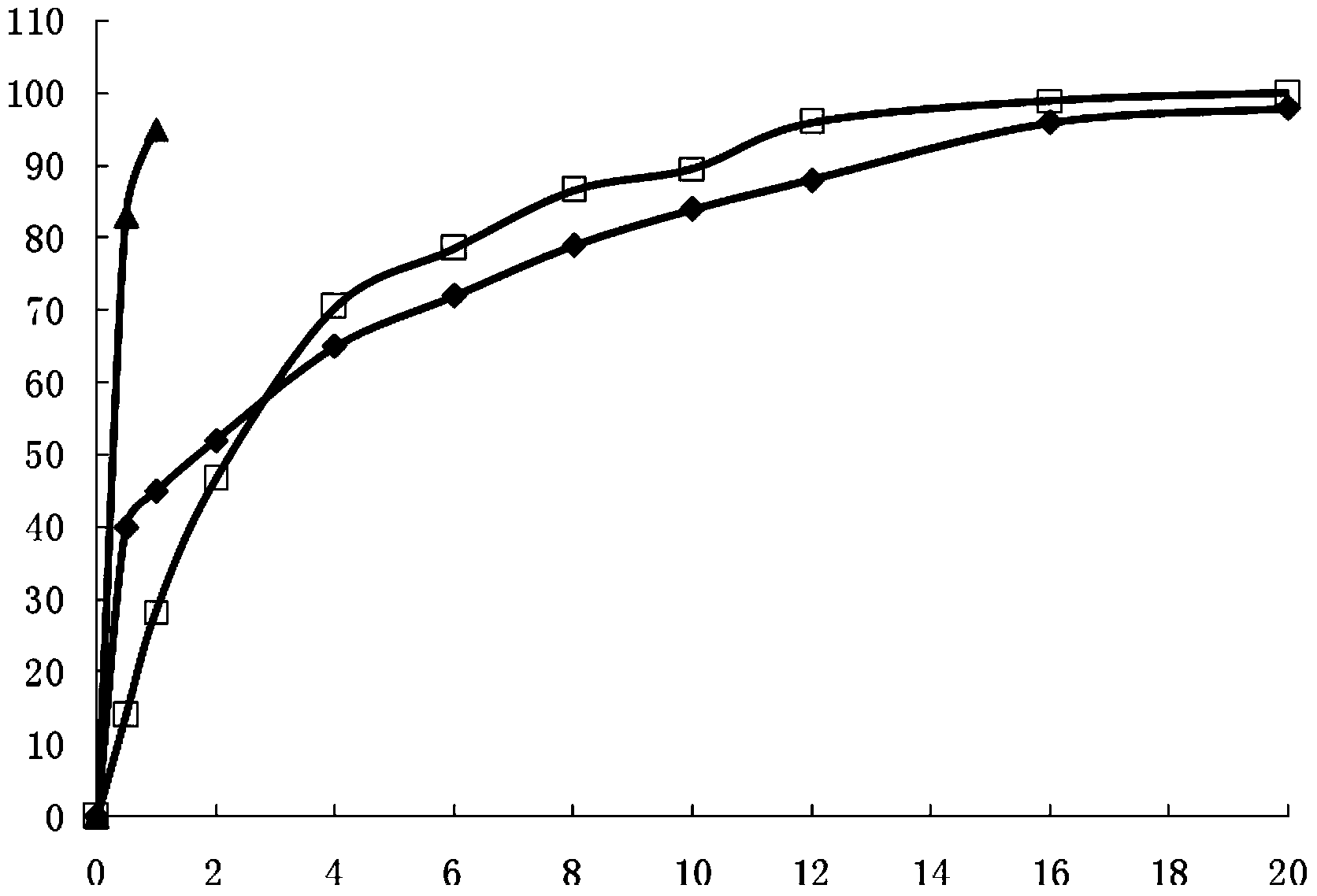
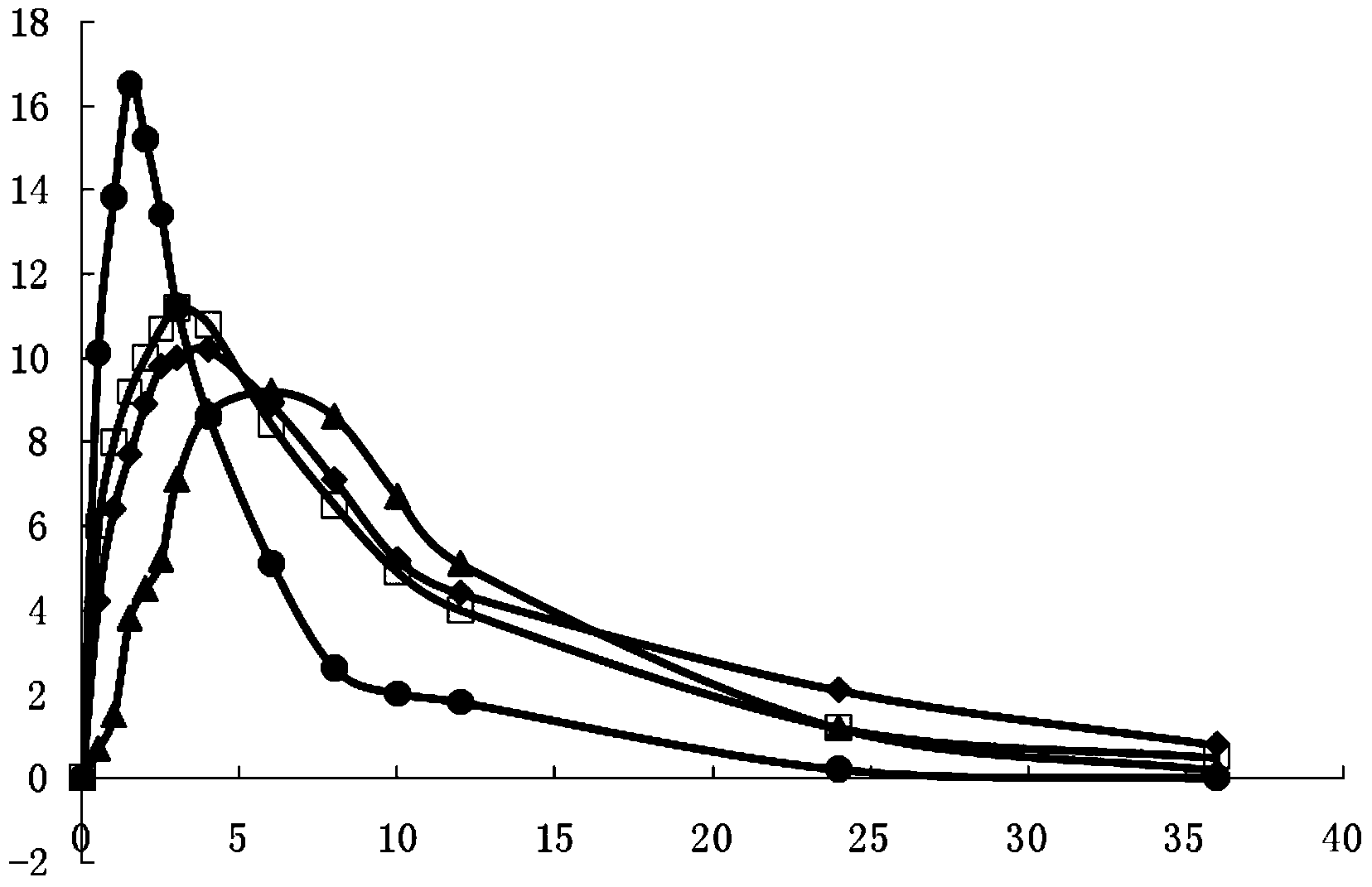
[0066] According to Chinese Pharmacopoeia Dissolution Determination Method (appendix XC first method), with phosphate buffer (pH7.2) 900ml as dissolution medium, rotating speed is 100 revolutions per minute, the capsule produced in Example 1 is carried out dissolution detection, in The release rate at each set time point is as follows: 40% release in 30 minutes, 45% release in 1 hour, 65% release in 4 hours, 77% release in 8 hours, 88% release in 12 hours, 96% release in 16 hours, and 98% release in 20 hours , and compared it with the release of commercially available sustained-release tablets (Ibuprofen Sustained-release Tablets Shanghai Xinyi Pharmaceutical Co., Ltd.) and ordinary tablets (Ibuprofen Tablets Shanghai Xinyi Pharmaceutical Co., Ltd.). (See figure 1 )
Embodiment 3
[0068]
[0069]
[0070] Dissolve 15 grams of polyvinylpyrrolidone in the slow-release part in 60 grams of purified water to prepare a 20% aqueous solution, mix it with 500 grams of ibuprofen and 250 grams of microcrystalline cellulose, and use 20% polyvinylpyrrolidone solution in the rapid Granulate in a wet granulator, dry, and mix the dried granules after sieving, 150 grams of hypromellose, 6 grams of silicon dioxide, and 5 grams of magnesium stearate to make a slow-release matrix, which is set aside.
[0071] Dissolve 20 grams of polyvinylpyrrolidone in the immediate release part in 60 grams of purified water to form a 25% aqueous solution, mix 150 grams of microcrystalline cellulose and 300 grams of ibuprofen evenly, and use 25% polyvinylpyrrolidone solution in the rapid Granulate in a wet granulator SHK-4B, dry, mix the sieved dry granules, 40 grams of crospovidone, 4 grams of silicon dioxide, and 3 grams of magnesium stearate to make an immediate-release matrix, re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com