Pharmaceutical composition for treating psoriasis and preparation method thereof
A composition and psoriasis technology, applied in the field of medicine, can solve the problems of large toxic and side effects, poor tolerance of patients, etc., and achieve the effect of small side effects, slow onset of treatment, and definite curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
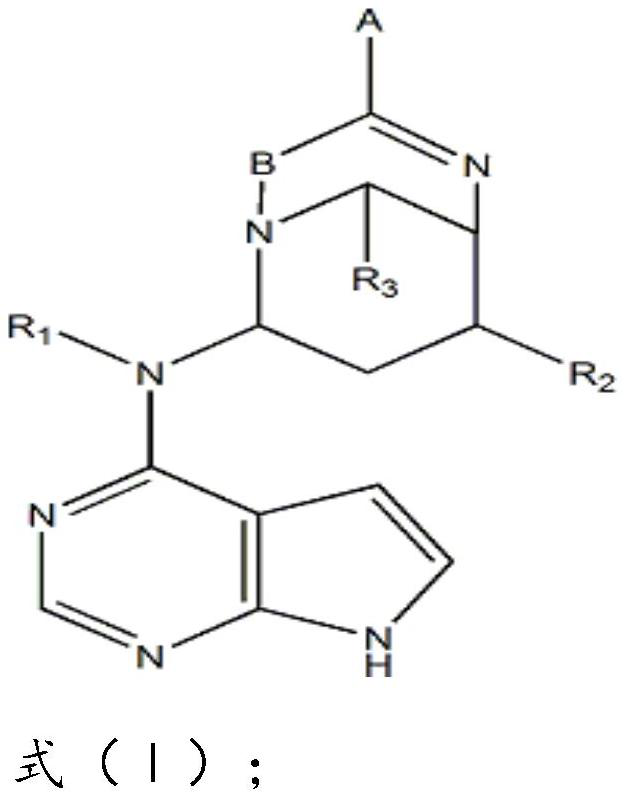
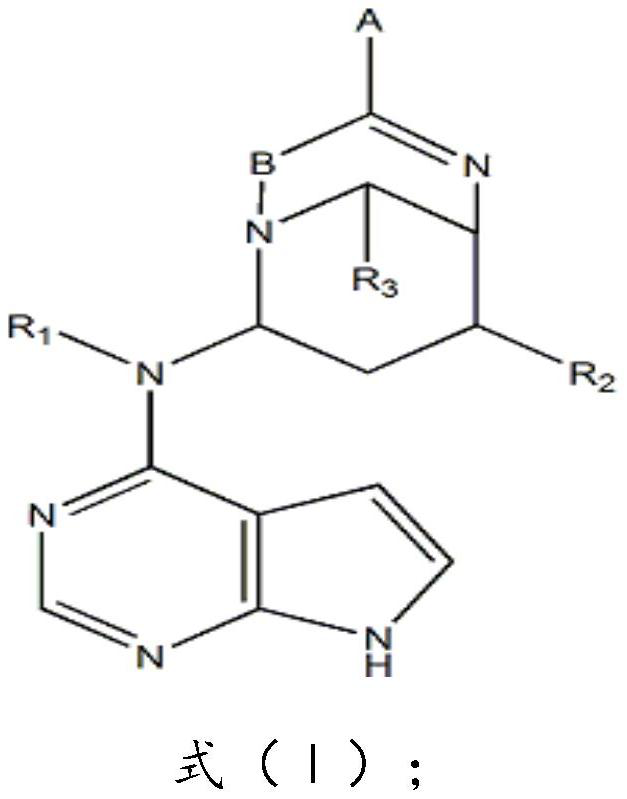
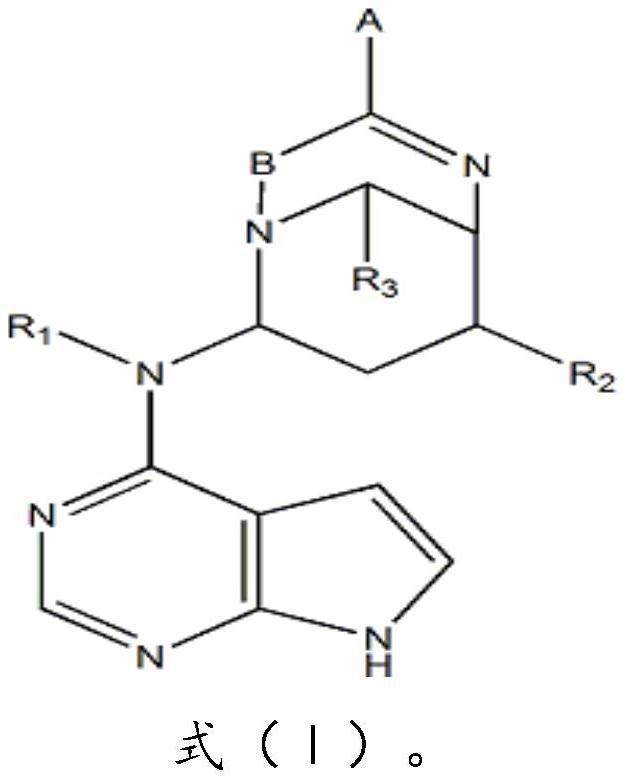
[0035] Compound (I) in this example, A is -COONa; B is C; R 1 for CH3; R 2 is OH; R 3 for H.
[0036] The pharmaceutical composition disclosed in this embodiment for the treatment of psoriasis comprises the following components and dosage:
[0037] Element Dosage / tablet Compound (Ⅰ) 10g ethanol 50g carbomer 10g Polysorbate 80 1.5g glycerin 30g Parabens 0.8g sodium hydroxide 2.5g Laurocaprazine 1.5g water Add to 1000g
[0038] This embodiment also discloses the preparation method of the above-mentioned pharmaceutical composition for treating psoriasis, comprising the following steps:
[0039] Step 1: Dissolving compound (I) in ethanol, then adding paraben and laurocaprazine and stirring evenly;
[0040] Step 2: Stir and dissolve the carbomer in 500g of water, dissolve the sodium hydroxide in 50g of water, then add the aqueous sodium hydroxide solution into the carbomer solution, and stir until it becomes ...
Embodiment 2
[0044] In the compound (I) of the present embodiment, A is -CF3; B is C; R 1 is OH; R 2 is OH; R 3 It is a C6 membered heterocycloalkyl group.
[0045] The pharmaceutical composition disclosed in this embodiment for the treatment of psoriasis comprises the following components and dosage:
[0046] Element Dosage / tablet Compound (Ⅰ) 10g ethanol 50g carbomer 10g Polysorbate 80 1.5g glycerin 30g Parabens 0.8g Triethanolamine 6g Laurocaprazine 1.5g water Add to 1000g
[0047] This embodiment also discloses the preparation method of the above-mentioned pharmaceutical composition for treating psoriasis, comprising the following steps:
[0048] Step 1: Dissolving compound (I) in ethanol, then adding paraben and laurocaprazine and stirring evenly;
[0049] Step 2: Stir and dissolve the carbomer in 500g of water, dissolve the triethanolamine in 50g of water, then add the triethanolamine aqueous solution into the ...
Embodiment 3
[0053] In the compound (I) of the present embodiment, A is -CF3; B is C; R 1 is OH; R 2 is OH; R 3 It is a C3 membered heterocycloalkyl group.
[0054] The pharmaceutical composition disclosed in this embodiment for the treatment of psoriasis comprises the following components and dosage:
[0055] Element Dosage / tablet Compound (Ⅰ) 10g Glyceryl monostearate 50g stearic acid 1.5g glycerin 80g paraffin 30g vaseline 50g Parabens 0.8g Poloxamer 5g Triethanolamine 6g Laurocaprazine 1.5g water Add to 1000g
[0056] This embodiment also discloses the preparation method of the above-mentioned pharmaceutical composition for treating psoriasis, comprising the following steps:
[0057] Step 1: The compound (I) is jet-pulverized to a particle size of ≤5 μm, and then added to glycerin to disperse evenly, then add paraben and laurocaprazine and stir evenly, then add vaseline and paraffin to disperse evenly; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com