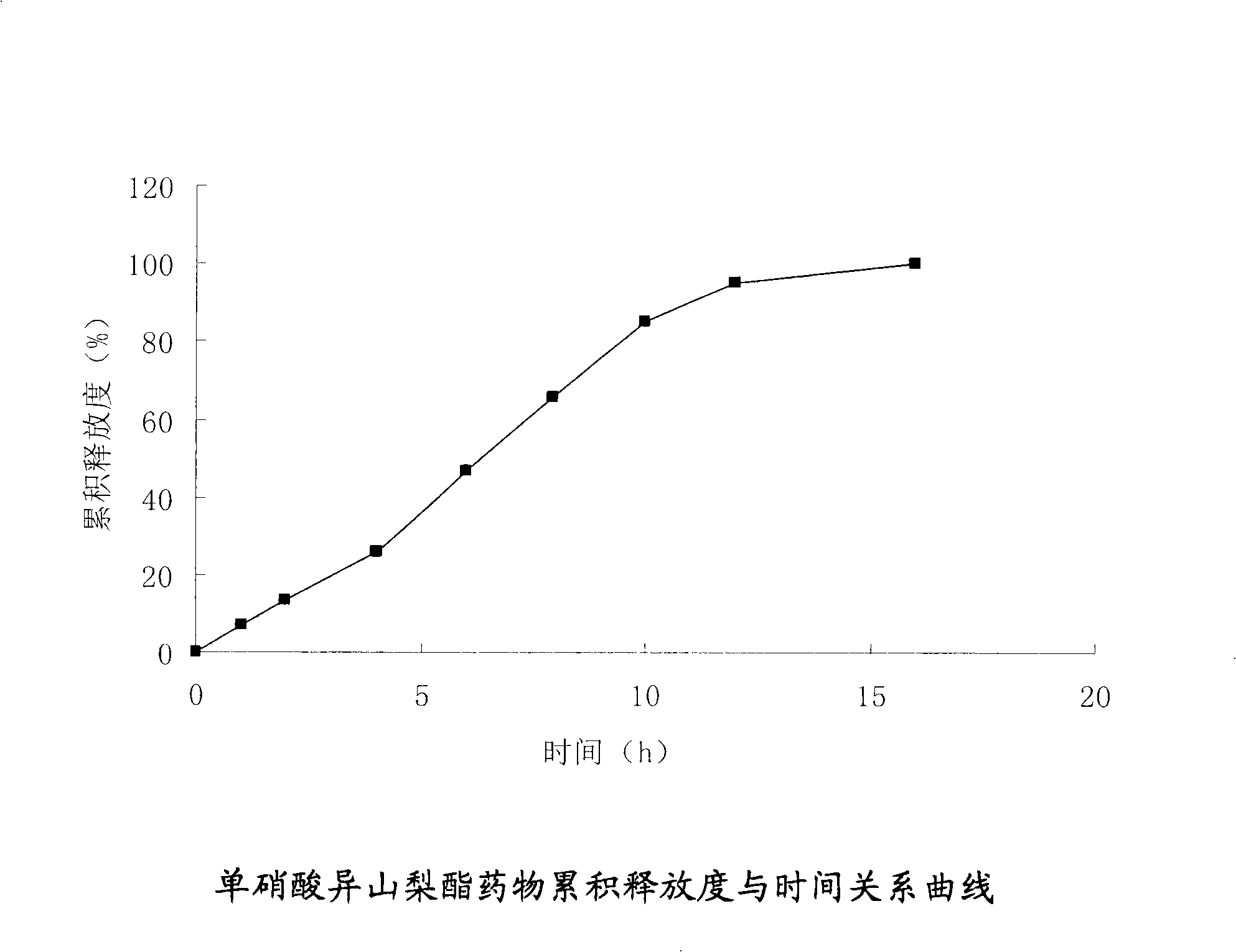
Isosorbide mononitrate osmotic pump type controlled release formulation and preparation method thereof
A controlled-release technology of isosorbide dinitrate and osmotic pumps, which is applied in the fields of pharmaceutical formulations, drug combinations, cardiovascular system diseases, etc., can solve problems such as difficulty in controlling the onset of angina pectoris in the morning, great influence of individual differences, and poor patient compliance, etc., to achieve Avoid peaks and valleys, easy to take, and long-lasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Prescription composition (based on 1000 tablets)
[0035] Chip:
[0036] Isosorbide Mononitrate 60g
[0038] Lactose 135g
[0039] Povidone K 30 30g
[0042] Coating solution:
[0043] Ethylcellulose 50mg / ml
[0044] Hypromellose 4.5mg / ml
[0045] PEG4000 4.5mg / ml
[0046] Dichloromethane: ethanol 3:1 (V / V)
[0047] Isosorbide mononitrate, lactose, sodium chloride and povidone K 30 Sift and mix thoroughly. The soft material is prepared from a mixed solution of ethanol and water (95:5, volume ratio), granulated to 20 mesh, and dried in a 40°C oven. Sieve the dry granules with 18 meshes, add magnesium stearate and talcum powder, and press into tablet cores. Place in a coating pan for coating. Dry at 40°C for 16 hours to solidify the coating film. It is sufficient to prepare one or more small holes with a diameter of 0.1-1.0 mm on one side of the core b...
Embodiment 2
[0049] Prescription composition (based on 1000 tablets)
[0050] Chip:
[0051] Isosorbide Mononitrate 60g
[0052] Microcrystalline Cellulose 50g
[0053]Lactose 135g
[0055] Coating solution:
[0056] Cellulose acetate 40mg / ml
[0057] Diethyl phthalate 4.5mg / ml
[0058] PEG4000 8.0mg / ml
[0059] Acetone: water 95:5 (V / V)
[0060] Sieve isosorbide mononitrate, microcrystalline cellulose, and lactose, and mix thoroughly and evenly. The soft material is prepared from a mixed solution of ethanol and water (70:30, volume ratio), granulated with 20 mesh, and dried in a drying oven at 40°C. Sieve the dry granules with 18 mesh, add talcum powder and press into tablet cores. Place in a coating pan for coating. Dry at 40°C for 16 hours to solidify the coating film. It is sufficient to prepare one or more small holes with a diameter of 0.1-1.0mm on one side of the core by using a laser drilling machine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com