Cariprazine hydrochloride injection preparation, and preparation method and use thereof
A technology of cariprazine hydrochloride and injection preparations, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
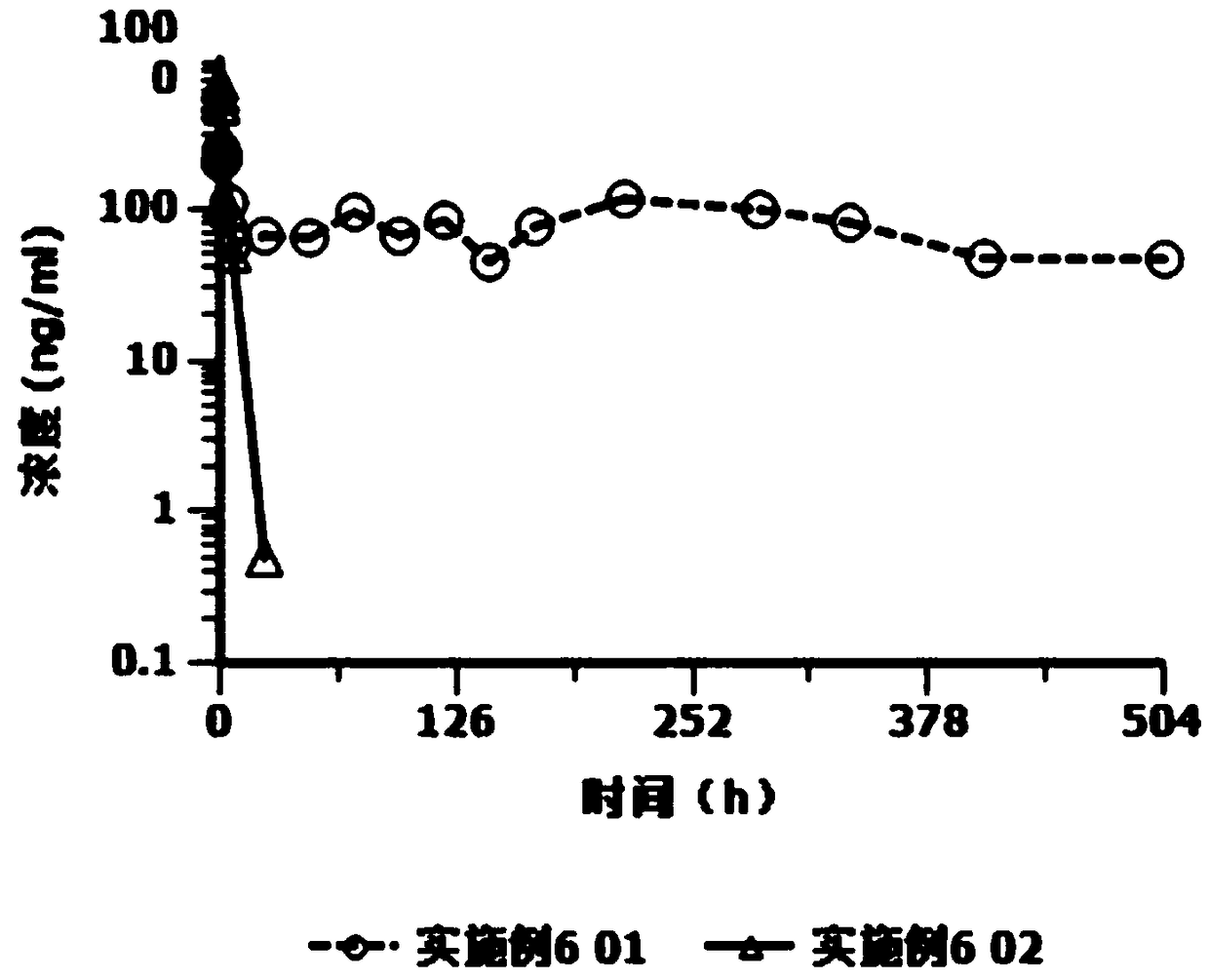
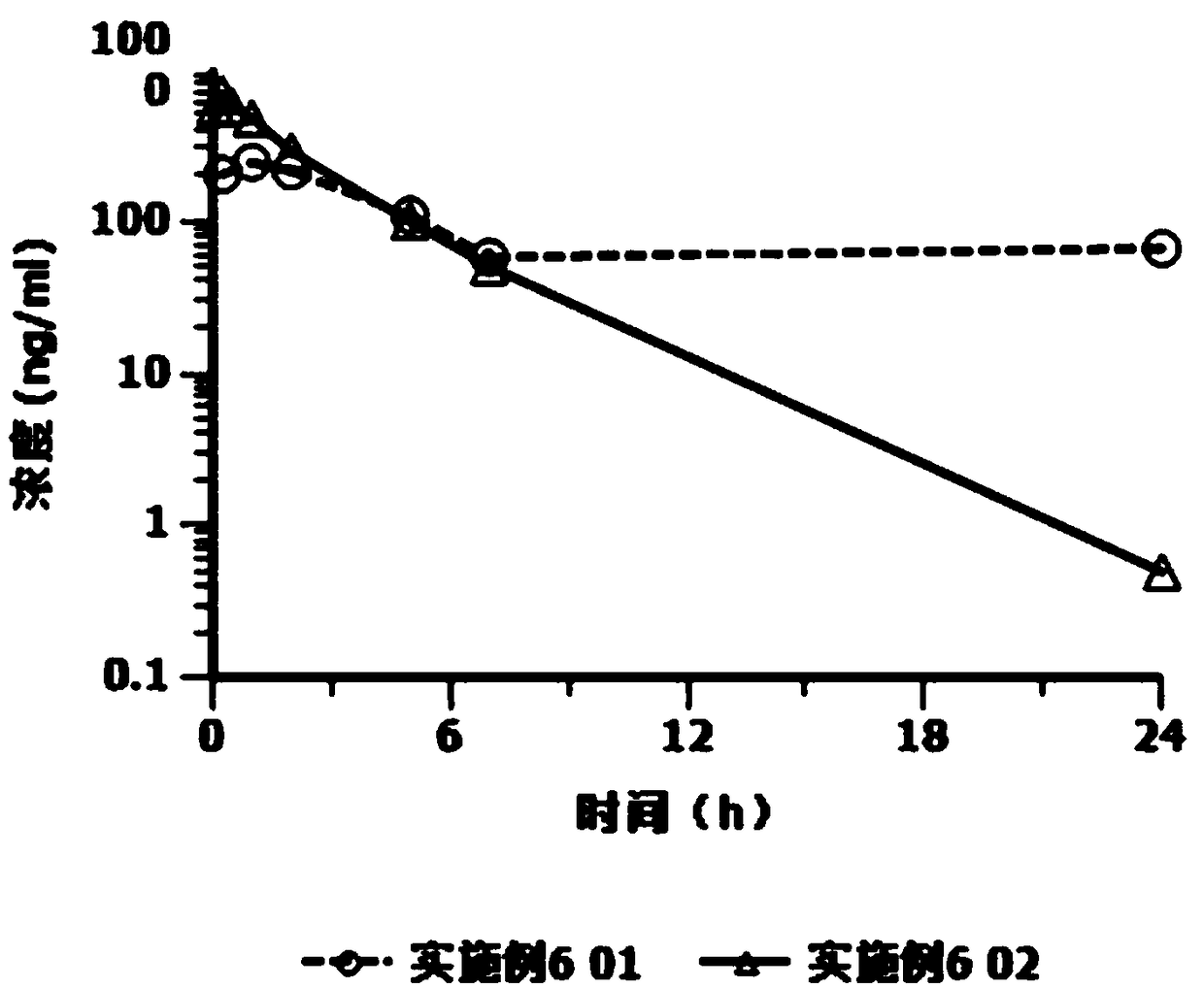
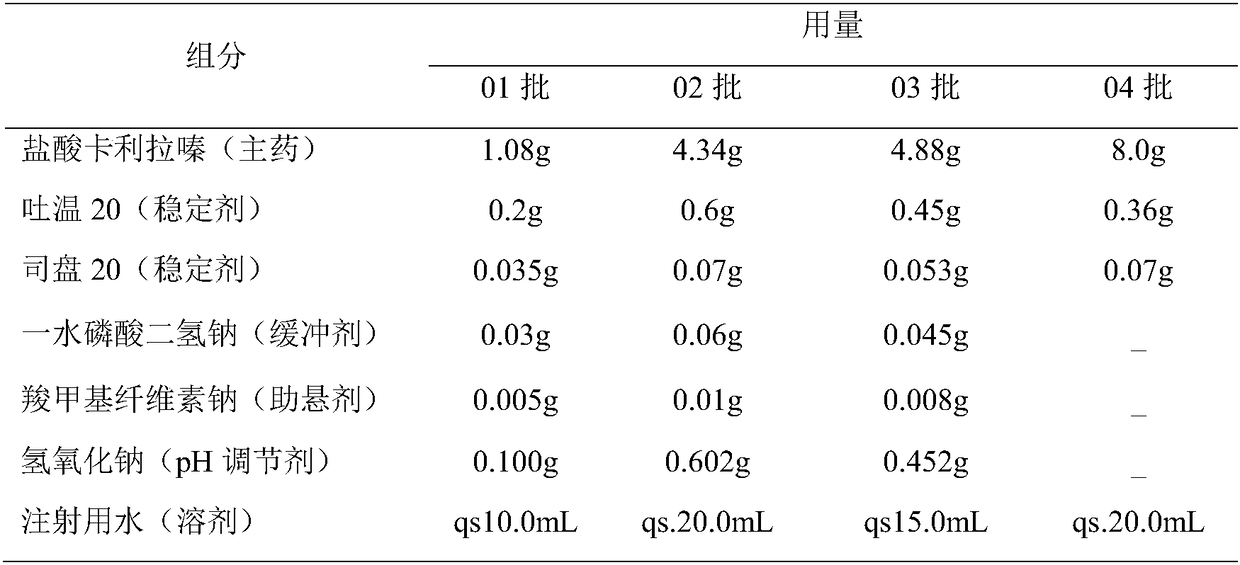
[0023] Based on the deficiencies of the prior art, the present invention has prepared an injection containing cariprazine hydrochloride after in-depth investigation and research. The preparation can be in the form of an aqueous suspension solution or a lyophilized preparation. The latter is mixed with matching sterile water for injection to form a suspension aqueous solution, and is used by intramuscular injection or subcutaneous injection. Compared with oral cariprazine hydrochloride capsules, the advantages of the invention include:
[0024] (1) Cariprazine hydrochloride exists in the granules with low solubility in the aqueous suspension solution, and the drug can be continuously released after injection, which can significantly reduce the number of administrations and avoid peak-to-trough fluctuations, thereby improving the patient's treatment compliance and safety;
[0025] (2) The drug loading of this preparation is relatively high, and a sustained release of at least 1 w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com