Preparation method for carbostyril injection
A quinolone and injection technology, which is applied in the field of preparation of quinolone long-acting injection, can solve the problems of inconvenient clinical medication, reduced dosage of administration, and reduced acupuncture, and can solve the phenomenon of peaks and troughs of blood drugs and prolong the release time. , the effect of prolonging the dosing cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation and in vitro evaluation of 10% enrofloxacin laurate injection
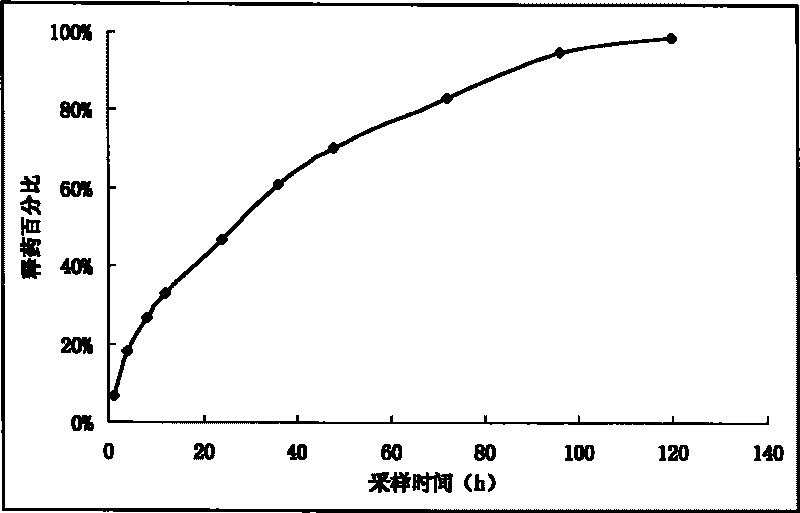
[0039] Add 10g of enrofloxacin (purified) and 5.6g of lauric acid into 50mL of propylene glycol, stir for 1h until the solution is clear, set the volume to 100mL with ethanol, add 0.3g of activated carbon, stir for 15min, and filter through a 0.45μm organic membrane to obtain 10% Enrofloxacin laurate injection. Take 1mL of injection and put it in a dialysis bag, dialysis conditions: 500mL of pH7.4PBS, 37°C, 100rpm. Respectively at 1h, 4h, 8h, 12h, 24h, 48h, 72h, 96h, 120h, until the concentration changes little. The drug release percentage-sampling time curve is shown as figure 1 As shown, the fitting results show that the in vitro release law of enrofloxacin laurate injection is best fitted by Higuchi equation, the in vitro release half-life is 27.2h, and the in vitro sustainable release is more than 120h.
Embodiment 2
[0041] Preparation of 30% Norfloxacin Stearate Injection
[0042] Take 40mL glycerin formal and 10mL propylene glycol, mix evenly, add 20g stearic acid, heat to 70°C during the stirring process, cool to room temperature after the stearic acid dissolves, add 30g norfloxacin (pure) in small amounts for several times, stir Until clarification, the volume of propylene glycol was adjusted to 100 mL, 0.3 g of activated carbon was added, stirred for 15 min, and filtered through a 0.45 μm organic membrane to obtain 30% norfloxacin stearate injection.
Embodiment 3
[0044] Preparation of 20% Compound Dafloxacin Oleate Injection
[0045] Add 20g of dafloxacin (purified) and 14.7g of oleic acid to 60mL of NMP, add 30g of sulfathiazole and 9.6g of benzyl alcohol, stir for 1h to a clear solution, set the volume of NMP to 100mL, add 0.3g of activated carbon, stir for 15min, 0.45 The 20% compound danofloxacin oleate injection was obtained by filtering with a μm organic membrane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com