Medicinal composition and application thereof
A composition and drug technology, applied in the direction of drug combination, antineoplastic drugs, pharmaceutical formulations, etc., can solve the problems that there is no literature to disclose liver cancer, no literature to report the synergistic effect of human endostatin and dexamethasone, etc., to achieve high resistance Active, highly effective preselected drugs for liver cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
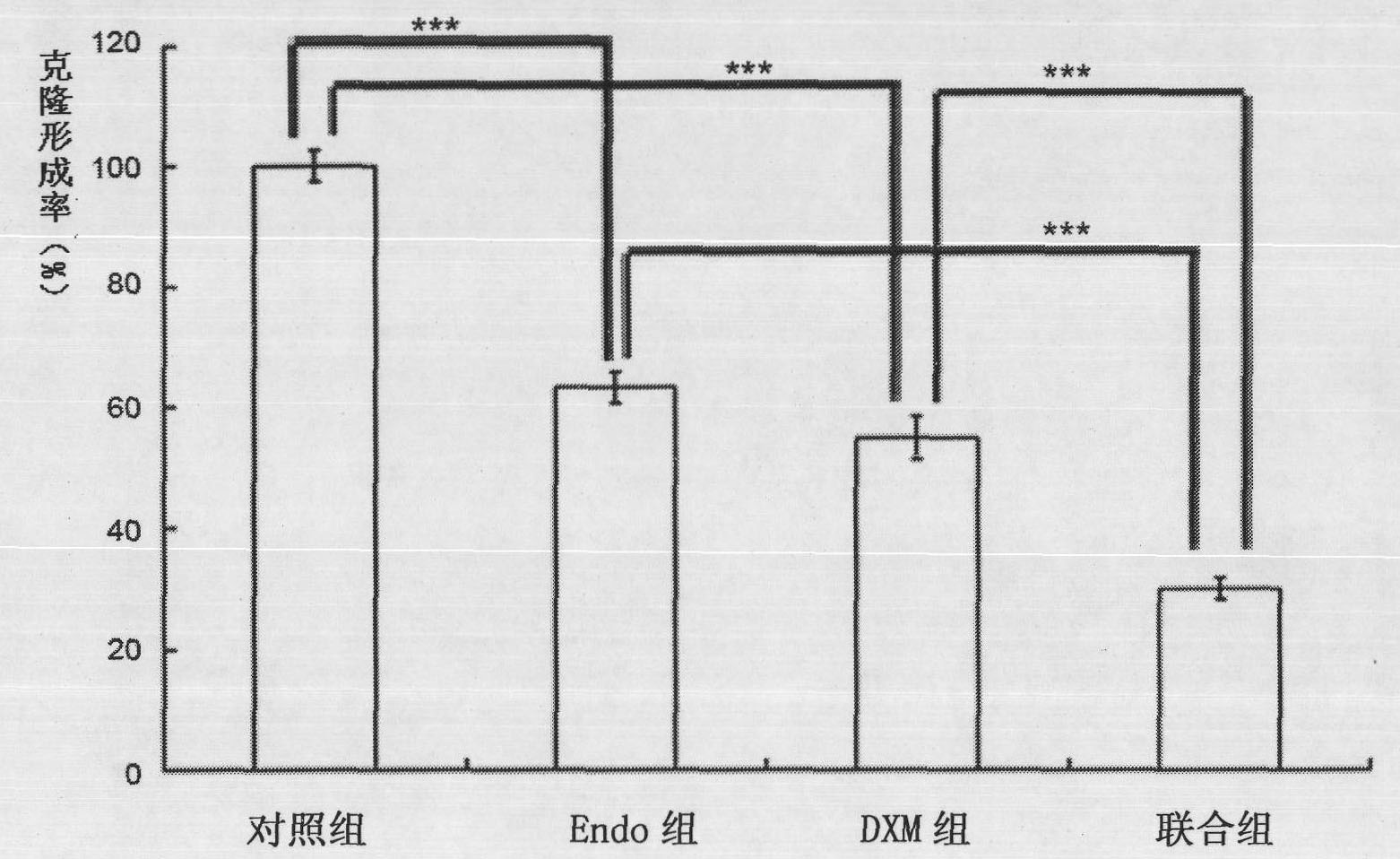
[0040] Embodiment 1 Clone formation analysis The influence of composition of the present invention on the proliferation of human umbilical vein endothelial cells (HUVEC)
[0041] Human umbilical vein endothelial cells (HUVECs) were diluted with ECM medium and inoculated in 24 wells (100cells / well), incubated at 37°C for 3 days and then treated with drugs. The experiment was divided into control group (Control, that is, serum-free ECM medium, The same below), recombinant human endostatin group (abbreviated as Endo group below and in the figure, recombinant human endostatin (abbreviated as Endo below): 12.5 μg / ml), dexamethasone sodium phosphate group (abbreviated as Endo below and in figure It is abbreviated as DXM group, dexamethasone sodium phosphate (hereinafter abbreviated as DXM): 0.5 μg / ml) and combination group (Endo12.5 μg / ml+DXM0.5 μg / ml). Continue to cultivate for 10 days, observe under the microscope and calculate the number of colony formation ( figure 1 ). The cl...
Embodiment 2
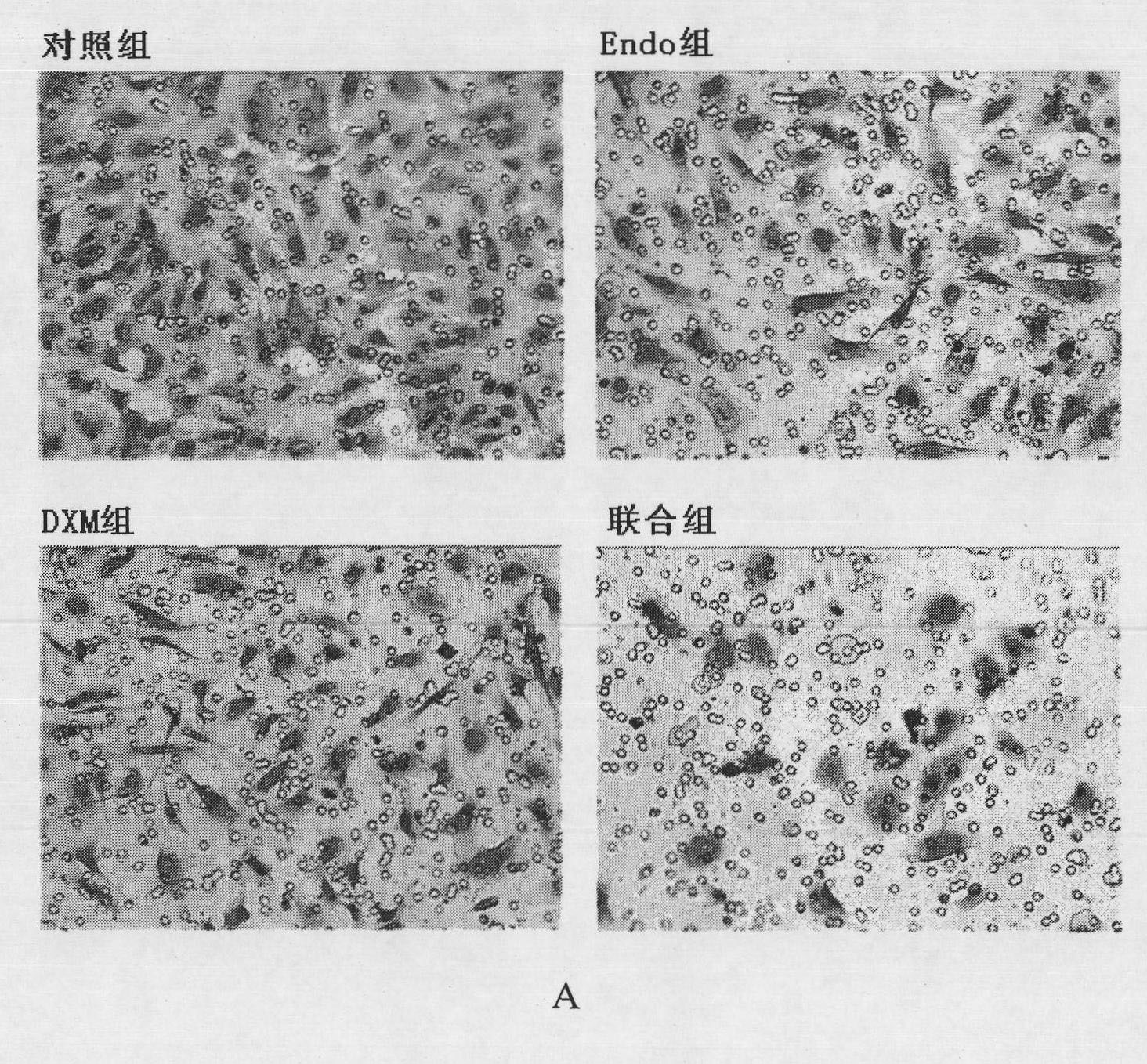
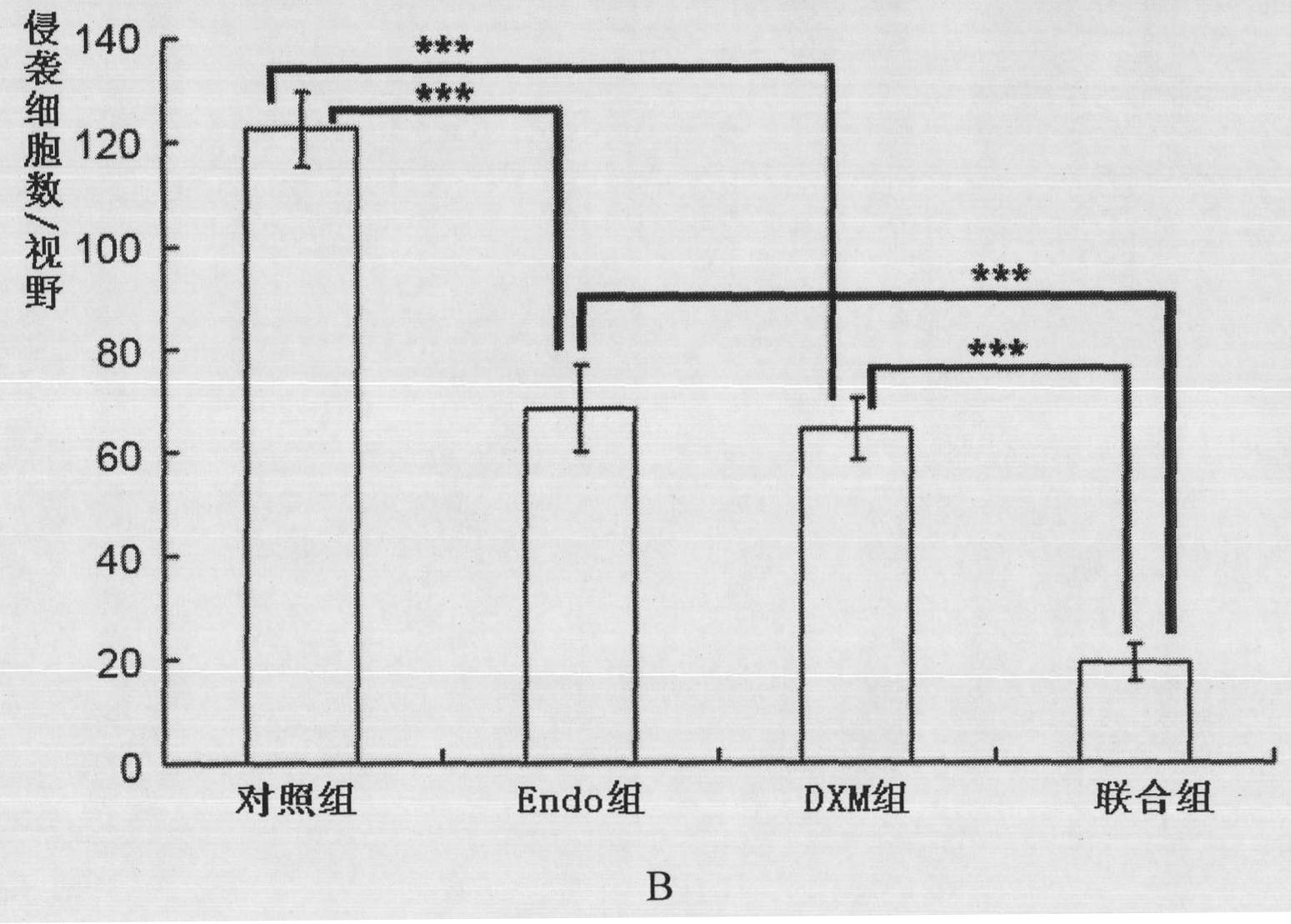
[0049] Example 2 Transwell method to analyze the effect of the composition of the present invention on the invasion of HUVECs
[0050] Place the Transwell chamber in a 24-well plate, equilibrate each chamber overnight with serum-free medium, then coat the upper layer of the chamber with Matrigel gel, inoculate HUVEC cells in the upper chamber of the Transwell, add ECM medium in the lower chamber, and then Adding drugs and treating at 37°C for 18 hours, the experiment was divided into control group (Control), Endo group (250 μg / ml), DXM group (5 μg / ml) and combined group (Endo250 μg / ml+DXM5 μg / ml). Take out the small chamber and stain with hematoxylin, erase the cells that have not passed through the membrane, take pictures and count the cells that have penetrated the membrane ( figure 2 ), and calculate the cell invasion rate and synergy index (CDI) according to the following formula:
[0051] Cell invasion rate = number of penetrating cells in the treatment group / number of ...
Embodiment 3
[0055] Example 3 Tube Formation Experiment Analysis of the composition of the present invention on the impact of HUVEC tubule formation
[0056] HUVEC cells were seeded in 100 μl Matrigel-coated 96-well plates (2×10 4 cells / well), and dosing treatment. The experiment was divided into control group (Control), Endo group (250μg / ml), DXM group (5μg / ml) and combination group (Endo250μg / ml+DXM5μg / ml). After incubation at 37°C for 24 hours, photographs were taken under an inverted microscope (×200), and the tubular structures formed by HUVEC cells were counted ( image 3 ), and calculate the inhibition rate and synergy index according to the following formula:
[0057] Inhibition rate=[1-(the number of tubules formed in the treatment group / the number of tubules formed in the control group)]×100%;
[0058] CDI=AB / (A×B), A=the number of tube formation in Endo group / the number of tube formation in control group, B=the number of tube formation in DXM group / the number of tube formatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com