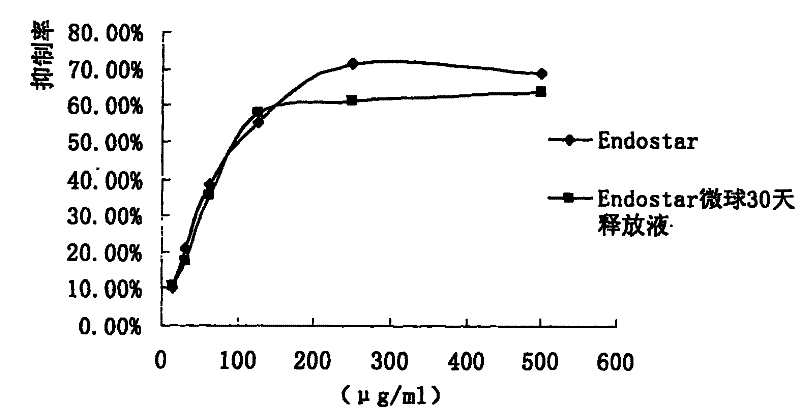
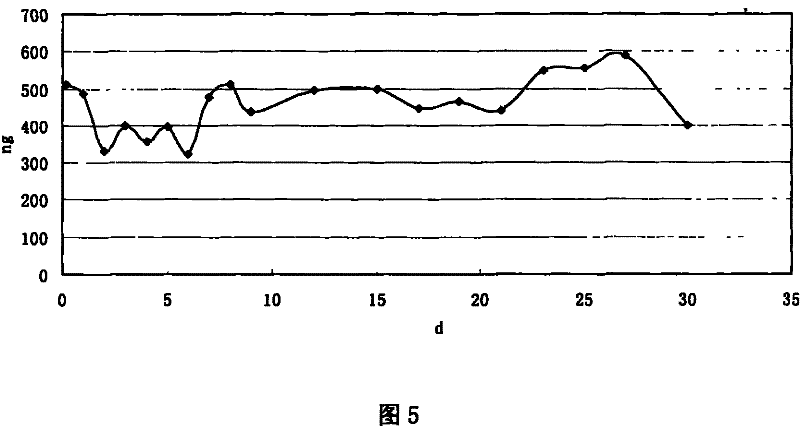
Preparation method of recombined human blood-vessel endothelia inhibin sustained-released microsphere
A technology of vascular endothelium and slow-release microspheres, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations containing active ingredients. High sealing rate and improved emulsification efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Accurately weigh 400 mg of Endostar and dissolve in 1 ml of PBS buffer (containing 3% gelatin) at pH 7.4 to form an internal aqueous phase. Dissolve 400 mg of PLGA (Mw=20000, 75:25) in 8 ml of a mixed solvent of dichloromethane and acetonitrile (1:1) to form an internal oil phase. Add the above internal water phase to the internal oil phase, disperse and emulsify at a high speed at 4000rpm to form W / O colostrum, then pour this colostrum into soybean oil containing 0.3% lecithin and 0.1% sucrose ester, and mechanically stir to evaporate the solvent (500rpm) for 4 hours, use 0.8 μm organic microporous membrane to filter to obtain microspheres, wash with petroleum ether three times, and finally freeze-dry to obtain the finished microspheres. Dissolve the finished microspheres in dichloromethane, extract the drug with PBS solution with pH=7.4, measure the protein concentration in the extracted solution by HPLC, and obtain the encapsulation efficiency of the microspheres. T...
Embodiment 2
[0044] Precisely weigh 2000mg Endostar and 200mg chondroitin sulfate and dissolve in 10ml Tris (10Mm PH7.4), adjust the pH value of the solution to 6.5-8.3, and measure the zeta potential at 0-48mv to form a chondroitin sulfate-Endostar complex thing. Take 0.5ml of the complex solution as the inner aqueous phase. 400 mg of PLGA (Mw=20000, 50:50) was dissolved in 8 ml of a mixed solvent of dichloromethane, acetonitrile and ethyl acetate (0.5:1:0.5) to form an internal oil phase. Add the above internal water phase to the internal oil phase, disperse and emulsify at a high speed at 4000rpm to form W / O colostrum, then pour this colostrum into liquid paraffin containing 0.8% Span-80 and 0.35% sucrose ester, and mechanically stir The solvent was evaporated (500 rpm) for 4 hours, and the microspheres were obtained by filtering with a 0.8 μm organic microporous membrane, washed three times with petroleum ether, and finally freeze-dried to obtain the finished microspheres. Dissolve t...
Embodiment 3
[0047] Accurately weigh 400mg of Endostar and dissolve in 1ml of sodium acetate solution (containing 5% bovine serum albumin) at pH=5.5 to form an inner aqueous phase. Respectively PLGA (A: Mw=12000, 50:50), (B: Mw=20000, 50:50), (C: Mw=40000, 50:50), (D: Mw=80000, 100:0) , (E: Mw=20000, 75:25), (F: Mw=130000, 75:25) 400mg is dissolved in the mixed solvent of 8ml dichloromethane and acetonitrile and ethyl acetate (0.5:1:0.5), becomes inner oil phase. Add the above-mentioned internal water phase to the above-mentioned internal oil phase, disperse and emulsify at a high speed at 4000rpm to form W / O colostrum, then pour this colostrum into soybean oil containing 0.5% lecithin and 0.2% sucrose ester, and mechanically stir The solvent was evaporated (500 rpm) for 4 hours, and the microspheres were obtained by filtering with a 0.8 μm organic microporous membrane, washed three times with petroleum ether, and finally freeze-dried to obtain the finished microspheres. Dissolve the fin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com