Preparation method of recombined human blood-vessel endothelia inhibin sustained-released microsphere
A technology of vascular endothelium and slow-release microspheres, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations containing active ingredients, etc., can solve the problems of drug leakage and low encapsulation rate, and achieve the reduction of activity loss and encapsulation The effect of high rate and narrow particle size distribution range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
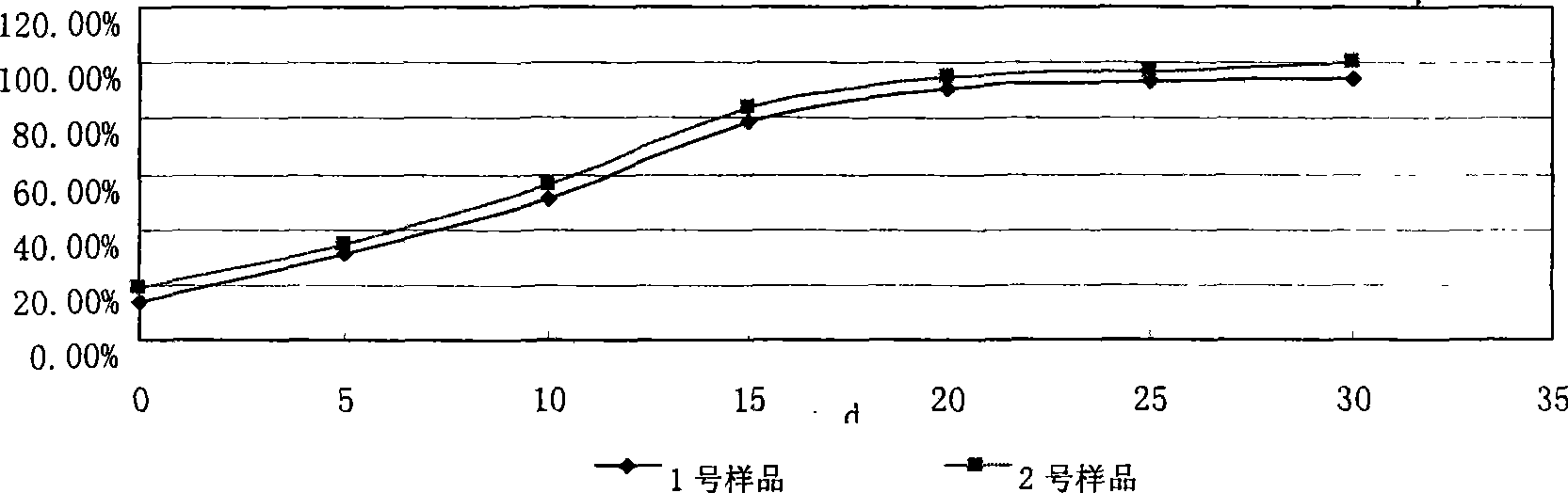
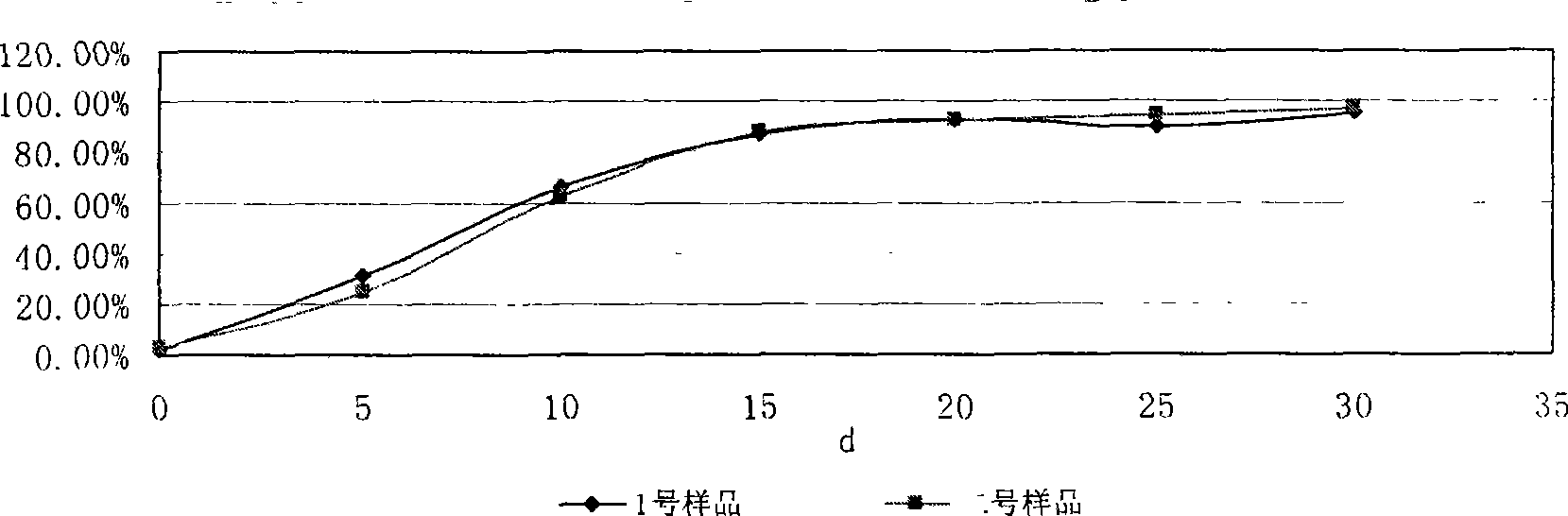
Image
Examples
Embodiment 1
[0041] 400 mg of Endostar was accurately weighed and dissolved in 1 ml of PBS buffer at pH 7.4 (containing 3% gelatin) to form an inner aqueous phase. 400 mg of PLGA (Mw=20000, 75:25) was dissolved in 8 ml of a mixed solvent of dichloromethane and acetonitrile (1:1) to obtain an inner oil phase. Add the above-mentioned inner water phase to the inner oil phase, disperse and emulsify at high speed at 4000 rpm to form W / O colostrum, then pour this colostrum into soybean oil containing 0.3% lecithin and 0.1% sucrose ester, and mechanically stir to evaporate the solvent (500rpm) for 4 hours, use a 0.8 μm organic microporous membrane to filter to obtain microspheres, wash three times with petroleum ether, and finally freeze-dry to obtain the finished microspheres. The finished microspheres were dissolved in dichloromethane, the drug was extracted with a PBS solution of pH=7.4, and the protein concentration in the extracted solution was measured by HPLC to obtain the encapsulation ef...
Embodiment 2
[0044] Accurately weigh 2000mg Endostar and 200mg Chondroitin Sulfate and dissolve it in 10ml Tris (10Mm PH7.4), adjust the pH of the solution to 6.5-8.3, and measure when its zeta potential is between 0 and -48mv to form a chondroitin sulfate-Endostar complex. thing. Take 0.5 ml of the complex solution as the inner water phase. 400 mg of PLGA (Mw=20000, 50:50) was dissolved in 8 ml of a mixed solvent of dichloromethane, acetonitrile and ethyl acetate (0.5:1:0.5) to obtain an inner oil phase. Add the above-mentioned inner water phase to the inner oil phase, disperse and emulsify at high speed at 4000 rpm to form W / O colostrum, then pour this colostrum into liquid paraffin containing 0.8% Span-80 and 0.35% sucrose ester, stir mechanically The solvent was evaporated (500 rpm) for 4 hours, and the microspheres were obtained by filtration with a 0.8 μm organic microporous membrane, washed three times with petroleum ether, and finally freeze-dried to obtain the finished microspher...
Embodiment 3
[0047] Accurately weigh 400 mg of Endostar and dissolve it in 1 ml of sodium acetate solution with pH=5.5 (containing 5% bovine serum albumin) to form an inner aqueous phase. PLGA (A: Mw=12000, 50:50), (B: Mw=20000, 50:50), (C: Mw=40000, 50:50), (D: Mw=80000, 100:0) , (E: Mw=20000, 75:25), (F: Mw=130000, 75:25) 400 mg were dissolved in 8 ml of a mixed solvent of dichloromethane, acetonitrile and ethyl acetate (0.5:1:0.5) to become Internal oil phase. Add the above-mentioned inner water phase to the above-mentioned inner oil phase respectively, disperse and emulsify at high speed at 4000 rpm to form W / O colostrum, then pour this colostrum into soybean oil containing 0.5% lecithin and 0.2% sucrose ester, and stir mechanically. The solvent was evaporated (500 rpm) for 4 hours, and the microspheres were obtained by filtration with a 0.8 μm organic microporous membrane, washed three times with petroleum ether, and finally freeze-dried to obtain the finished microspheres. The fini...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com