Novel boric acid derivative and medicinal composition
A boronic acid and compound technology, applied in the field of anti-tumor compounds and their pharmaceutical compositions, can solve problems such as unmet clinical needs of therapeutic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Example 1 Preparation of compound I-1-1 of the present invention and its mannitol ester:
[0168]
[0169] (1) Dissolve 3 g (17.6 mmol) of the starting material (S-1-1) in 20 mL of dichloromethane solution, add 4.44 g (44.0 mmol) of NMM (N-methylmorpholine), and cool down to an internal temperature of 5 ℃, drop 2.29 g (21.1 mmol) of ethyl chloroformate, wait until the reaction is complete, and use the reaction solution for later use.
[0170] (2) Glycine 1.58g (21.1mmol) is dissolved in 30ml saturated sodium carbonate solution, is cooled to internal temperature 5 ℃, and step (1) gained reaction solution is added dropwise in this saturated sodium carbonate solution of glycine, internal temperature change control Within ±2°C, after the dropwise addition was completed, continue to stir at room temperature for 3h. Take the water layer, adjust the pH value to 2-3 with 15ml of dilute hydrochloric acid (2mol / ml), a solid precipitates, filter with suction, take the solid, a...
Embodiment 2
[0178] Example 2 Preparation of Compounds I-1-2, I-1-2-1, I-1-2-2, I-1-2-3, I-1-2-4 of the present invention
[0179]
[0180] Boric acid starting material (I-1-1) 5 grams (14.7mmol), diethanolamine (M2, molecular weight 105.14) 1.58 grams (15.0mmol), 20mL ethyl acetate, stirred overnight at room temperature, precipitated a white solid, filtered to obtain 4.8 gram compound I-1-2, yield 79%, namely 2-chloro-5-methyl-[(R)-1-[1,3,7,2]-dioxazepine-2-boryl- 3-Methyl-butyrocarboxamido]-methyl]-benzamide.
[0181] 1 H NMR (300 MHz, DMSO-d6) δ (ppm): 8.69 (brs, 1H), 7.35-7.39 (m, 2H), 7.25-7.29 (m, 1H), 6.95 (d, 1H, J=4.71Hz ), 6.59(m, 1H), 3.74-3.79(m, 2H), 3.67(m, 3H), 3.57(m, 1H), 3.13(m, 1H), 2.99(m, 2H), 2.73-2.78( m, 2H), 2.31 (m, 3H), 1.59 (m, 1H), 1.18-1.30 (m, 2H), 0.81 (m, 6H).
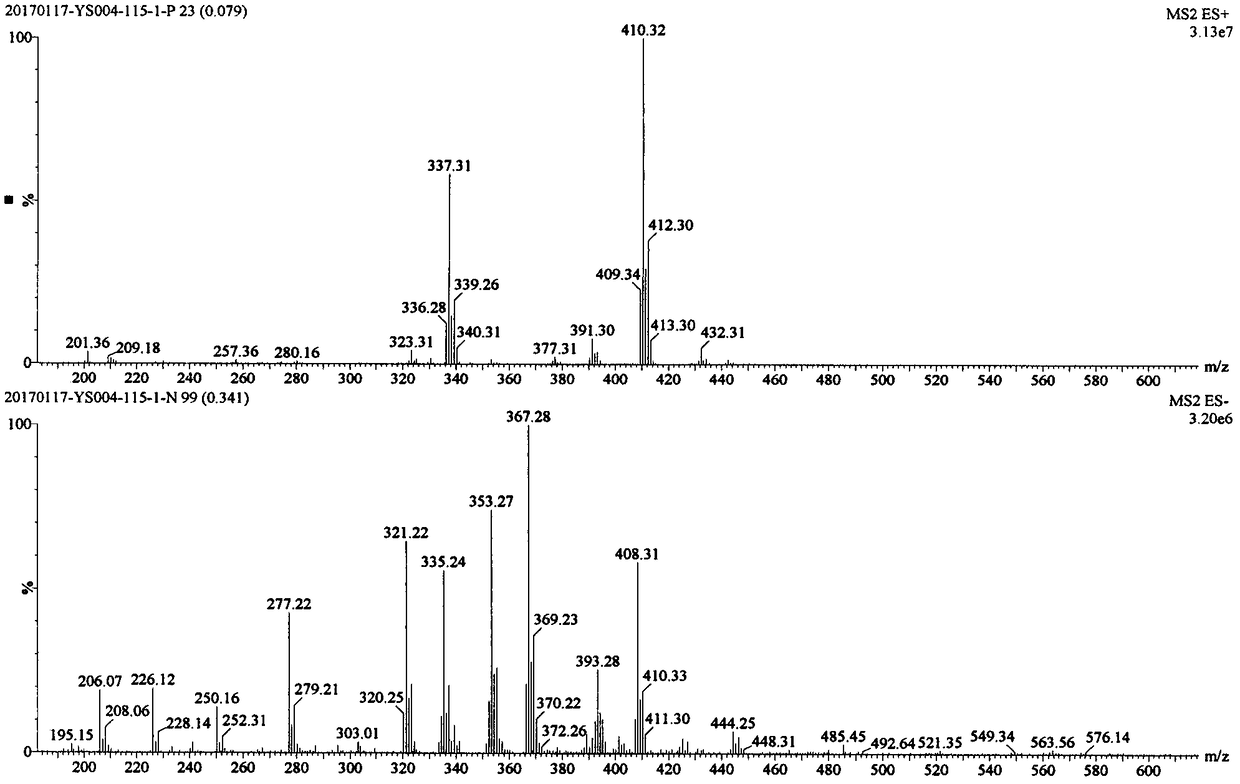
[0182] ESI m / z: 410.32[M+H] + .
[0183] The mass spectrum of compound I-1-2 is as followsfigure 1 shown.
[0184] Similar to the synthesis of compound I-1-2, the boronic acid starting mat...
Embodiment 3
[0194] Example 3 Preparation of compounds I-1-3 and I-1-3-1 of the present invention
[0195]
[0196] Dissolve 2.25 g (6.61 mmol) of boric acid starting material (I-1-1) in 10 ml of ethyl acetate, add 0.92 g (6.94 mmol) of dipropanolamine (M3, molecular weight 133.19) under room temperature stirring, and continue stirring for 2 h. A precious solid was precipitated, and 2.05 g of compound I-1-3 was obtained by filtration, with a yield of 71%.
[0197] ESI m / z: 436.42[M-H] - .
[0198] Similar to the synthesis of compound I-1-3, I-1-3-1 was prepared by reacting boronic acid starting material (I-1-1) with M3-1 tripropanolamine.
[0199]
[0200] ESI m / z: 518.31[M+Na] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com