Purification method of human immunoglobulin for intravenous injection
A technology of human immunoglobulin and purification method, which is applied in the field of biopharmaceuticals, can solve problems such as side effects and allergic reactions, and achieve the effects of increasing sample loading, reducing production time limit, and easy to scale up production operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0050] 1. Test materials and instruments: strong anion exchanger, ultraviolet spectrophotometer, pH meter.
[0051] 2. Kits for the detection of IgA and IgM protein content: immunoglobulin A enzyme-linked immunosorbent assay kit; immunoglobulin M enzyme-linked immunosorbent assay kit.
[0052] 3. Detection of total protein content: biuret method
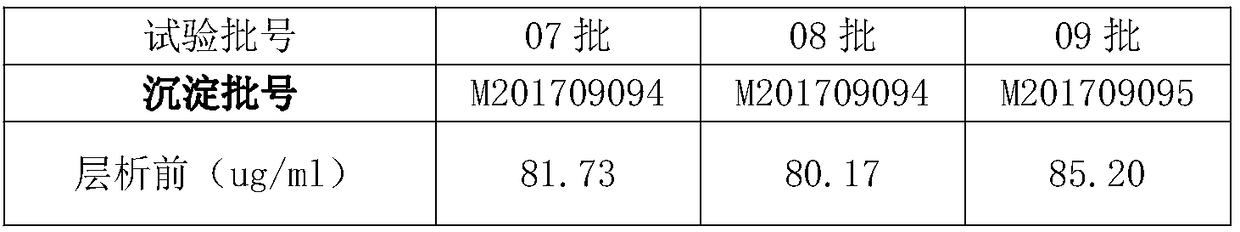
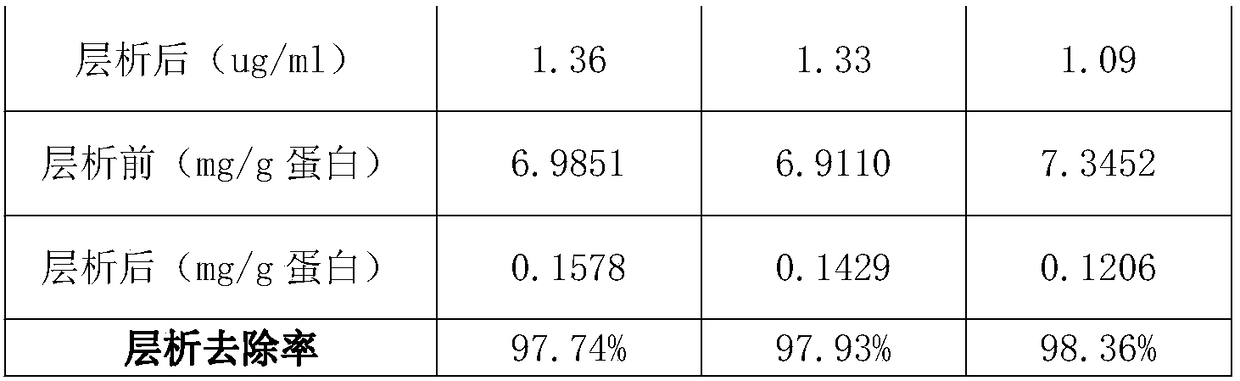
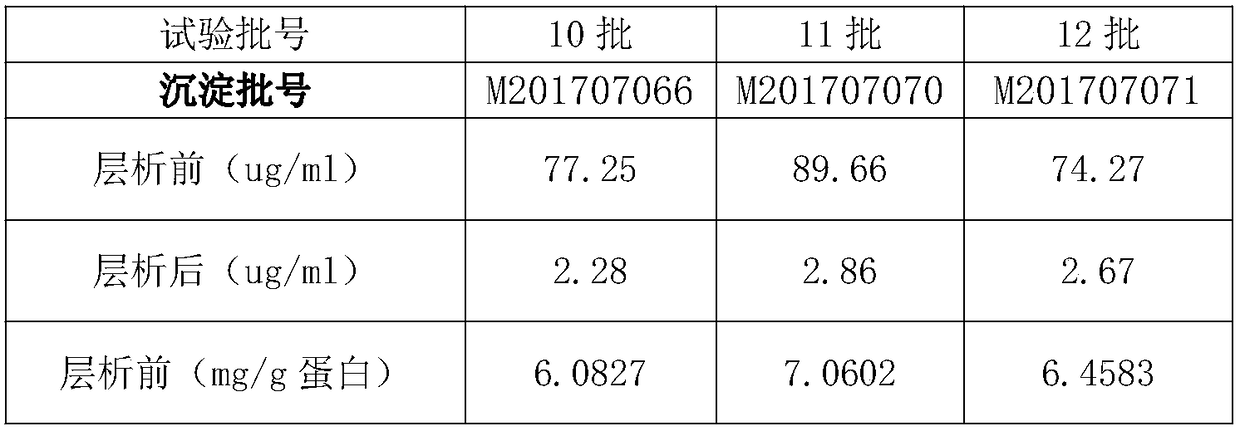
[0053] 4、试验材料:乙醇沉淀法制得的二次沉淀组分:01~18批,批号分别为M201709093、M201709093、M201709094、M201708087、M201708092、M201709095、M201709094、M201709094、M201709095、M201707066、M201707070、M201707071、M201709093、 M201709093, M201707078, M201710096, M201710098, M201710099.
[0054] Note: The batch numbers of batches 01 and 02 are the same batch number, and the batch numbers of batches 13 and 14 are the same batch number. This is because the production volume of one batch is large in the previous low-temperature ethanol production process, which can be divided into multiple times for chromatography.
[0055] 5. Required reagents:
[0056] 0.5mol / L sodium hydro...
Embodiment 1
[0064] 1. Test object: Batch No. 01 is M201709093
[0065] 2. Test process:
[0066] S1 Dissolution: Take water for injection at 6°C which is equivalent to 8 times the volume of the secondary precipitation component of batch 01, and test the endotoxin in the water for injection. After the endotoxin is qualified, it is used to dissolve the secondary precipitation component of batch 01, and stir to dissolve. The speed of the stirrer is controlled so that there is no foaming, and the stirring is stopped after 3 hours to obtain a solution, which is then sampled for the first time, and the protein concentration, pH, and conductivity are detected.
[0067] S2 Filtration: Install filter elements with 0.45μm pore size and 0.2μm pore size in sequence in the series filter stack, and rinse the filter until it is qualified. Filter the solution, control the filtration pressure to 0.13MPa, collect the filtered solution, and wash the filter with water for injection at 4.0°C after completion...
Embodiment 2
[0073] 1. Test object: Batch 02, batch number M201709093
[0074] 2. Test process:
[0075] S1 Dissolution: Take water for injection at 6°C equivalent to 5 times the volume of the secondary precipitation component of batch 02, and test the endotoxin in the water for injection. After the endotoxin is qualified, it is used to dissolve the secondary precipitation component of batch 02, and stir to dissolve. The speed of the stirrer is controlled so that no foaming prevails, and the stirring is stopped after 2 hours to obtain a solution, which is then sampled for the first time, and the protein concentration, pH, and conductivity are detected.
[0076] S2 Filtration: Install filter elements with 0.45μm pore size and 0.2μm pore size in sequence in the series filter stack, and rinse the filter until it is qualified. Filter batch 02 of the solution, control the filtration pressure to 0.14MPa, collect the filtered solution, and wash the filter with 8.0°C water for injection to obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com