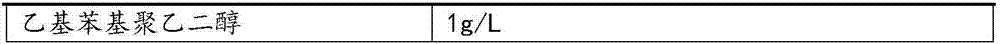Immunoglobulin A detection kit and detection method thereof
An immunoglobulin and detection kit technology, which is applied in biological testing, measuring devices, material testing products, etc., can solve the problems of inaccurate detection results of reagents, inability to meet the requirements for use, affecting the detection effect, etc., and achieve good stability of the reagents. , wide versatility, accurate detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] In order to enable those skilled in the art to better understand the technical solutions in the application, the present invention will be further described below in conjunction with the embodiments. Obviously, the described embodiments are only some of the embodiments of the application, not all of them. Based on the embodiments in this application, all other embodiments obtained by persons of ordinary skill in the art without creative efforts shall fall within the scope of protection of this application. Embodiment 1 Immunoglobulin A detection kit
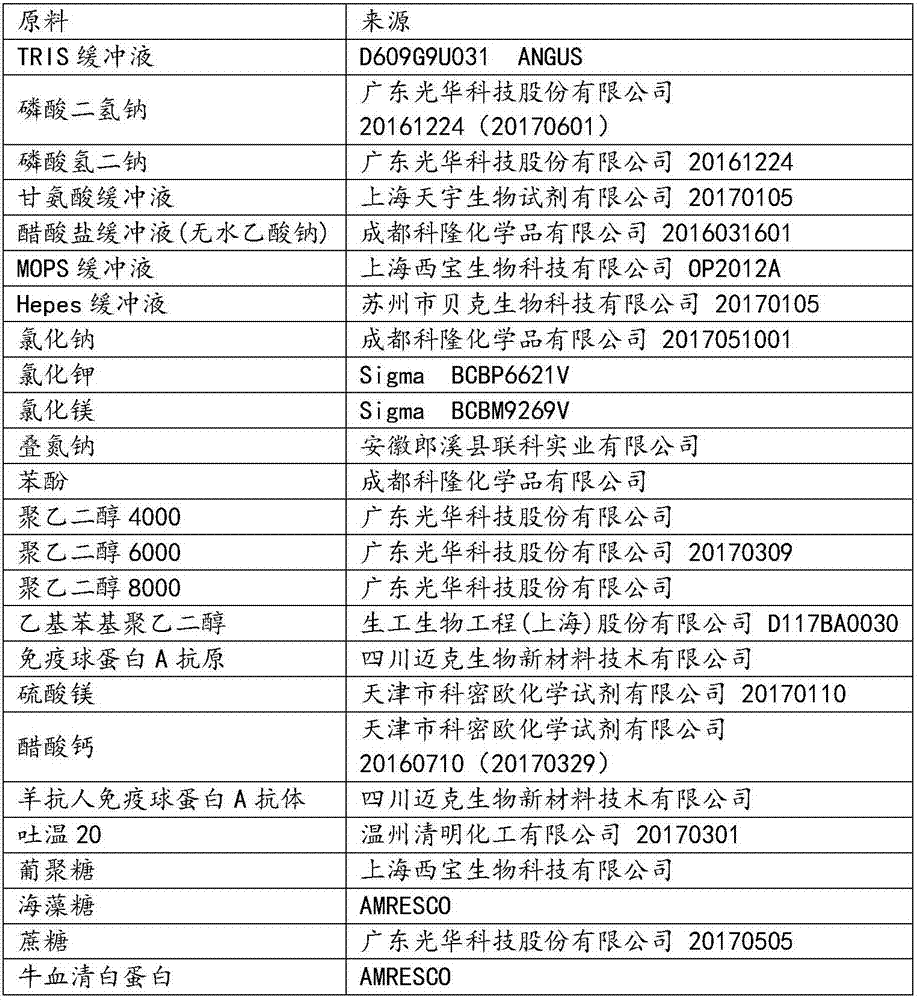
[0041] Reagent R1:
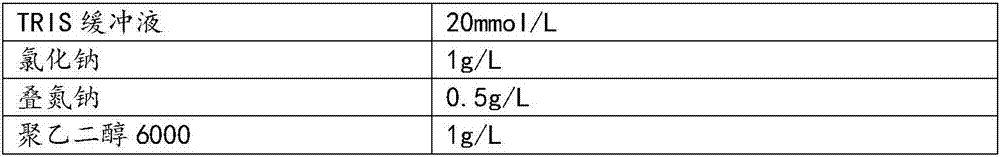
[0042] TRIS buffer
20mmol / L
1g / L
0.5g / L
polyethylene glycol 6000
1g / L
Ethylphenyl polyethylene glycol
1g / L
[0043] Reagent R2:
[0044] TRIS buffer
20mmol / L
1g / L
0.5g / L
Goat anti-human immunoglobulin A antibody
10mg / L
Ethylphenyl ...
Embodiment 2
[0047] Embodiment 2 Immunoglobulin A detection kit
[0048] Reagent R1:
[0049] Phosphate buffer
40mmol / L
7g / L
0.7g / L
polyethylene glycol 6000
30g / L
Ethylphenyl polyethylene glycol
50g / L
[0050] Reagent R2:
[0051] Phosphate buffer
40mmol / L
potassium chloride
5g / L
0.7g / L
Goat anti-human immunoglobulin A antibody
50mg / L
Ethylphenyl polyethylene glycol
50g / L
15.5g / L
calcium acetate
23g / L
[0052] Calibrator:
[0053] Immunoglobulin A antigen standard diluent (50mmol / L glycine buffer, 10g / L sodium chloride, 0.5g / L sodium azide, 20g / L dextran, 25g / L trehalose, 30g / L sucrose , 20g / L bovine serum albumin) was dissolved, tested with a commercially available control reagent and adjusted to 100mg / L, and stored in -20°C. Take it out before use, and dilute it with standard diluent to obt...
Embodiment 3
[0054] Embodiment 3 Immunoglobulin A detection kit
[0055] Reagent R1:
[0056] acetate buffer
80mmol / L
potassium chloride
20g / L
0.8g / L
polyethylene glycol 6000
45g / L
Ethylphenyl polyethylene glycol
5g / L
[0057] Reagent R2:
[0058] acetate buffer
80mmol / L
potassium chloride
20g / L
1g / L
Goat anti-human immunoglobulin A antibody
300mg / L
Ethylphenyl polyethylene glycol
5g / L
0.05g / L
calcium acetate
0.04g / L
[0059] Calibrator:
[0060] Immunoglobulin A antigen standard diluent (70mmol / L TRIS buffer, 25g / L sodium chloride, 1g / L sodium azide, 55g / L dextran, 50g / L trehalose, 60g / L sucrose, 75g / L bovine serum albumin) was dissolved, detected and adjusted to 200mg / L with a commercially available control reagent, subpackaged and stored at -20°C. Take it out before use, and dilute it with standa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com