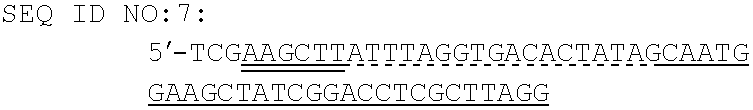
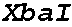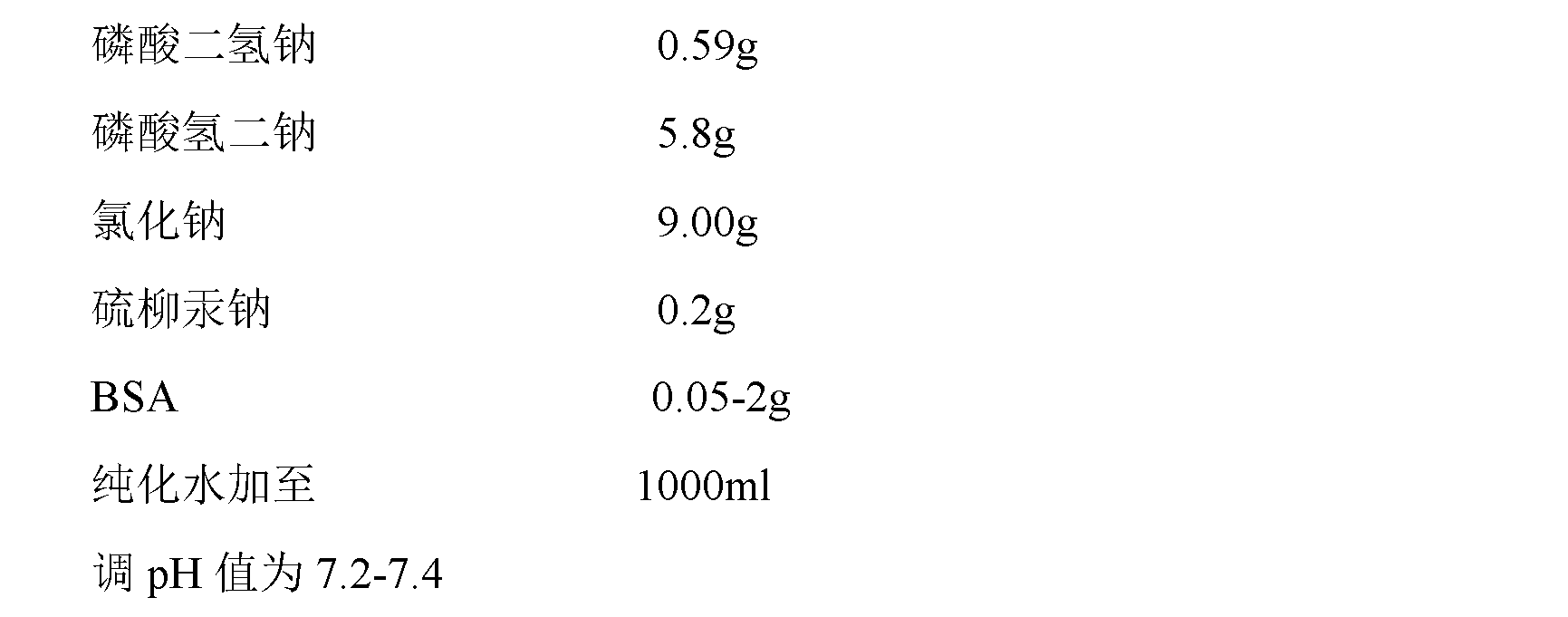Patents
Literature
92 results about "Rubella virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rubella virus (RuV) is the pathogenic agent of the disease rubella, and is the main cause of congenital rubella syndrome when infection occurs during the first weeks of pregnancy. Rubella virus is the only member of the genus Rubivirus and belongs to the family of Togaviridae, whose members commonly have a genome of single-stranded RNA of positive polarity which is enclosed by an icosahedral capsid.
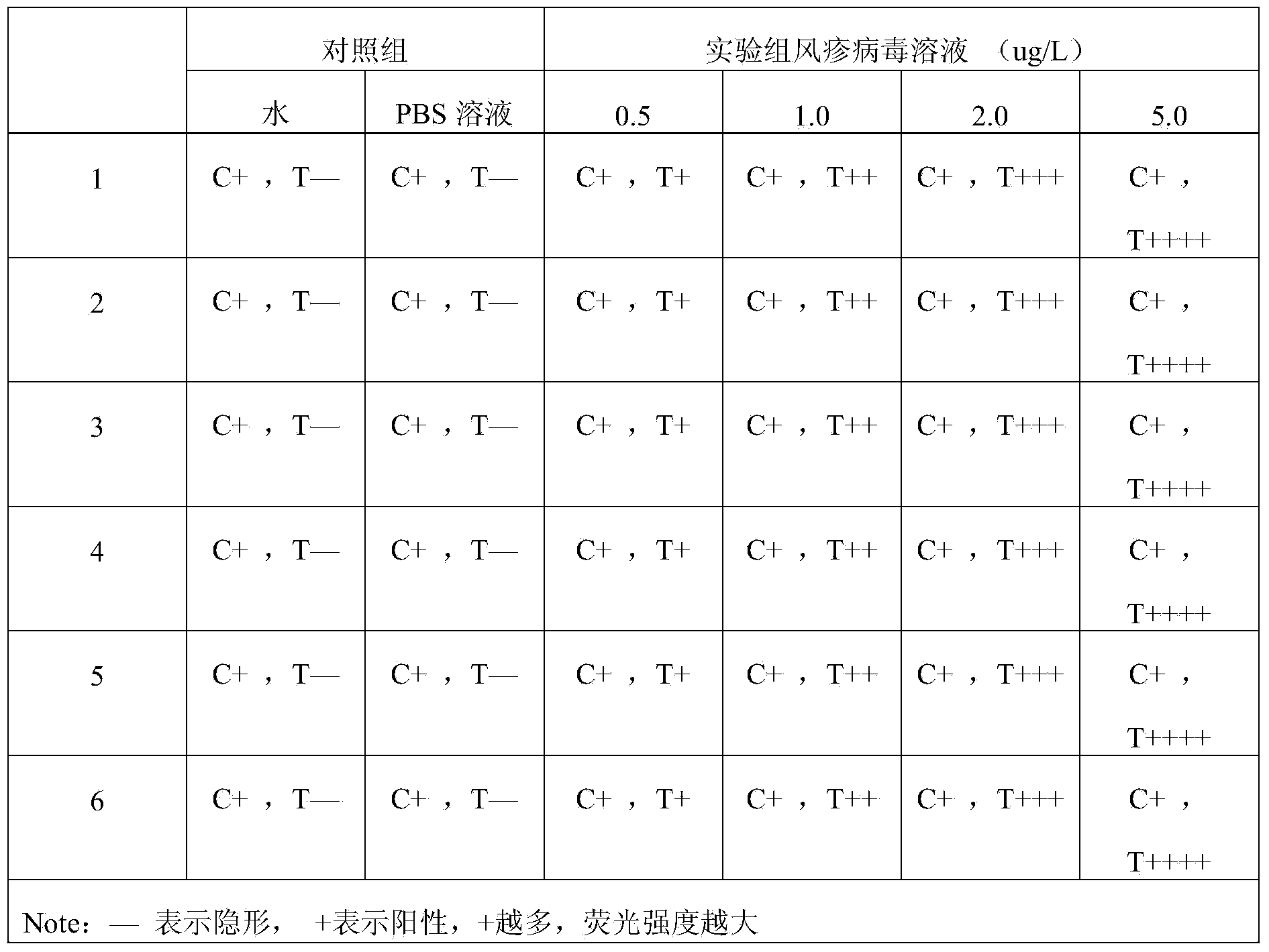
Multiple real-time quantitative PCR primer, probe and detection method for identifying viral pathogens relevant to fever with eruption syndrome as infection diseases
ActiveCN102140543ADetection ExpressImprove efficiencyMicrobiological testing/measurementFluorescence/phosphorescenceChickenpoxHerpes zoster virus
The invention discloses multiple real-time quantitative PCR primer, probe and a detection method for identifying viral pathogens relevant to fevers with eruption syndromes as infection diseases, which is used for carrying out multiple real-time fluorescent quantitative PCR detection on varicella-herpes zoster viruses, human small DNA (Deoxyribonucleic Acid) viruses B19, enteroviruses (enteroviruses 71 type and coxsackie viruses A16 type), dengue viruses, rubella viruses and measles viruses. The invention can simultaneously carry out qualitative or quantitative detection on eight kinds of human viruses in various types of samples by multiple double-tubes PCR. The detection method has the advantages of simple operation, short time consumption, high sensitivity and strong specificity, is suitable for field detection, early diagnosis, epidemics detection and research and the like, and takes the actions of assistance and identification diagnosis on the fevers with eruption syndromes.
Owner:SUN YAT SEN UNIV
Rubella virus IgG and IgM antibody joint inspection kit and preparation method thereof
InactiveCN102109519AQuick screeningEasy to operateMaterial analysis by observing effect on chemical indicatorGlass fiberNitrocellulose
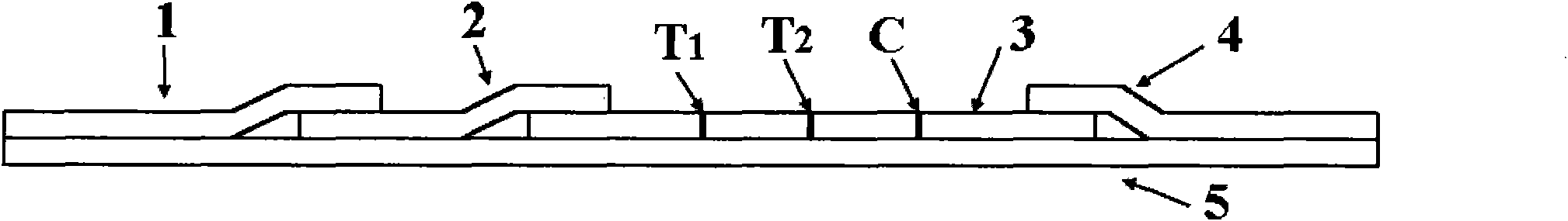
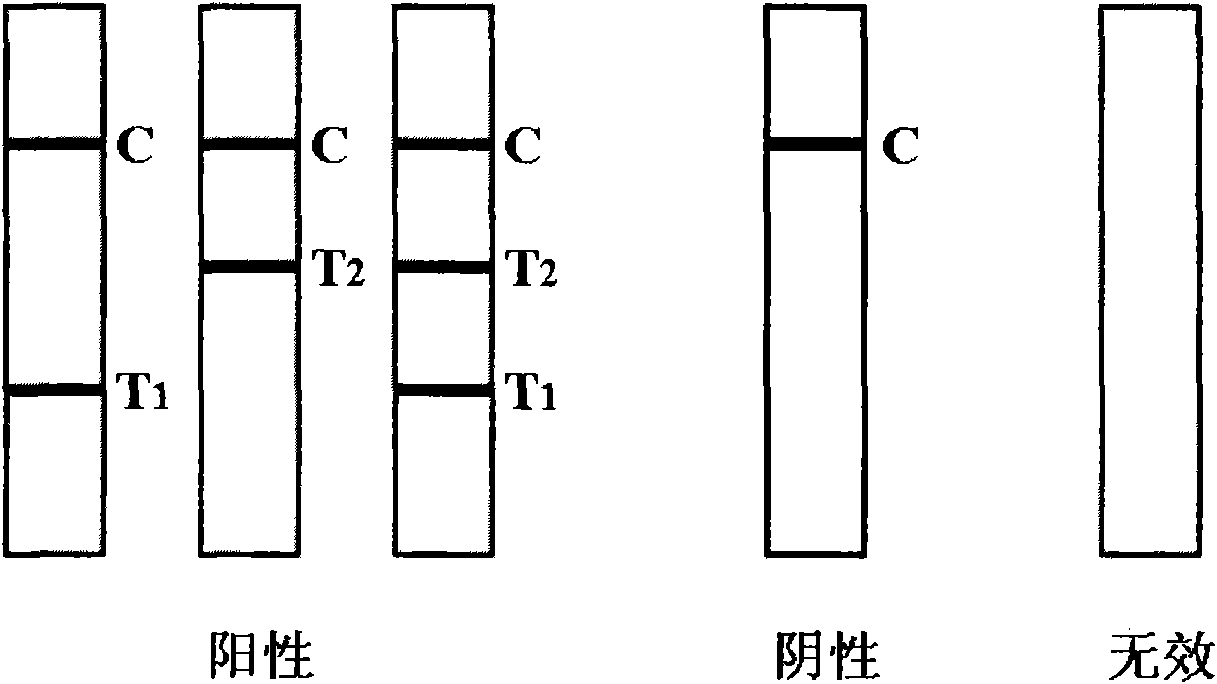
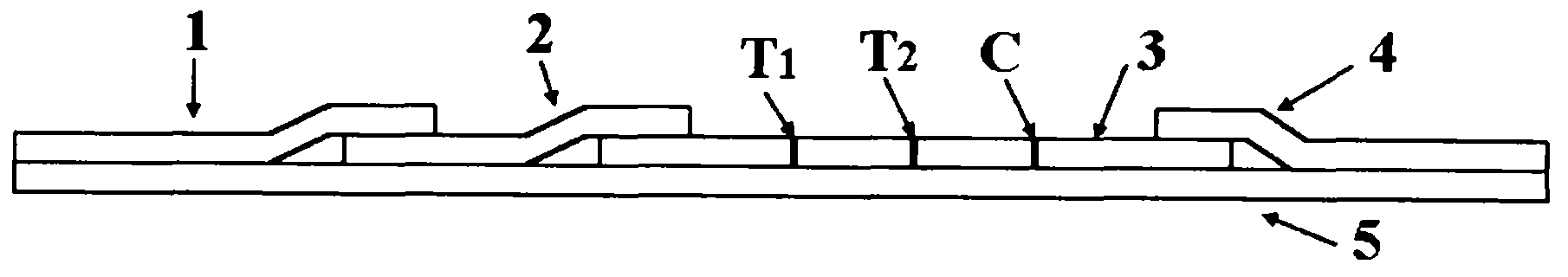
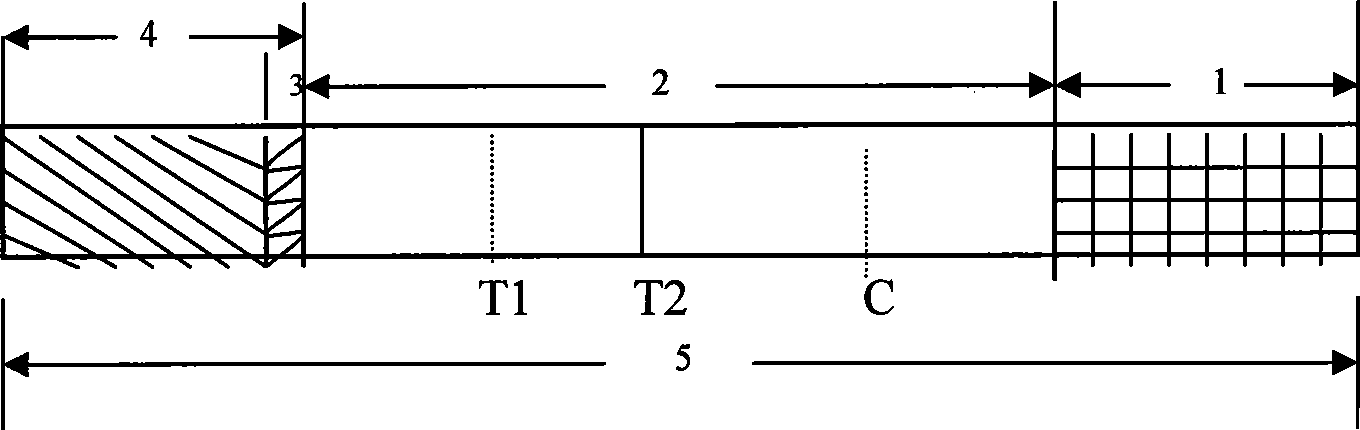
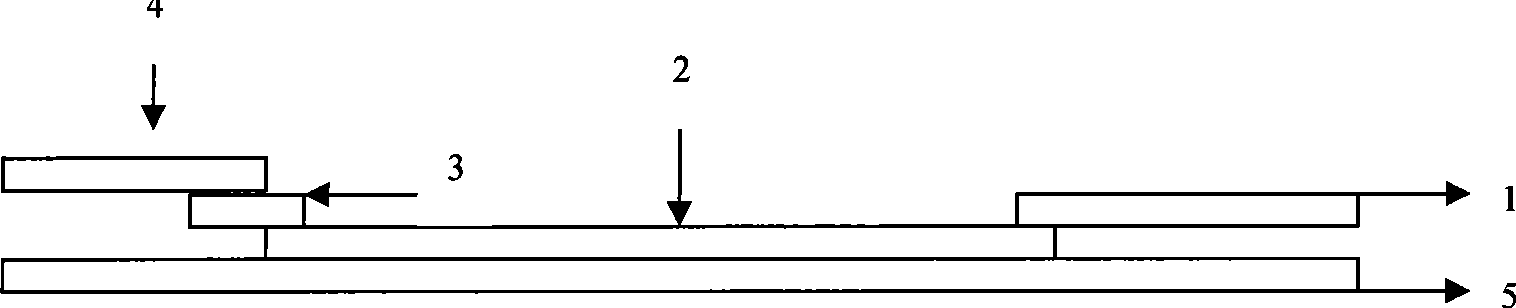
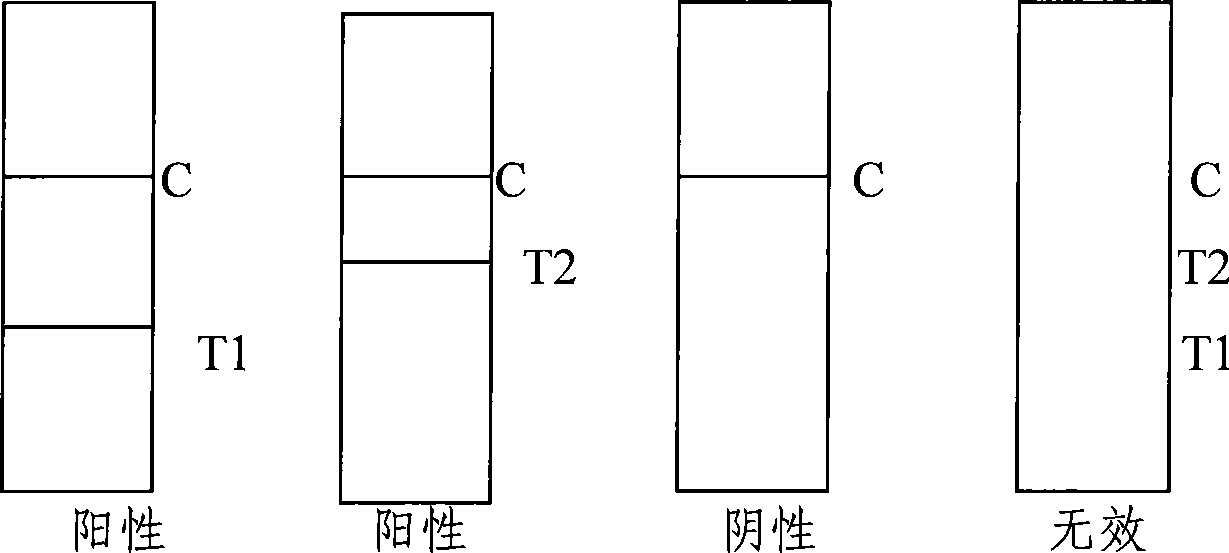
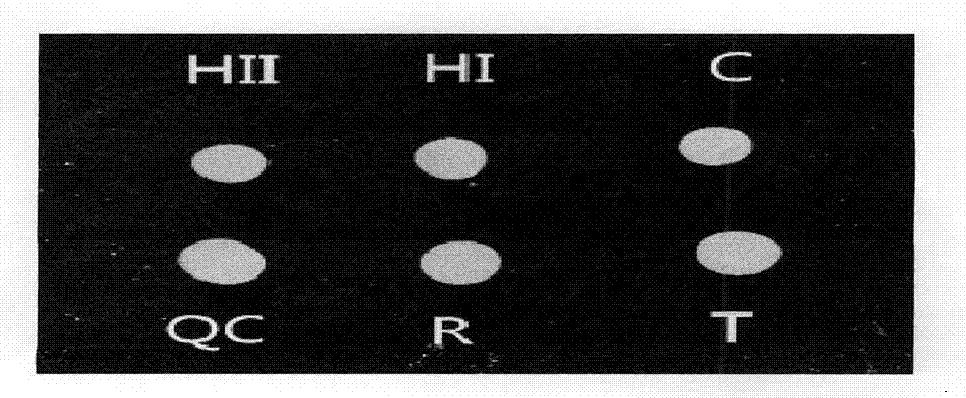
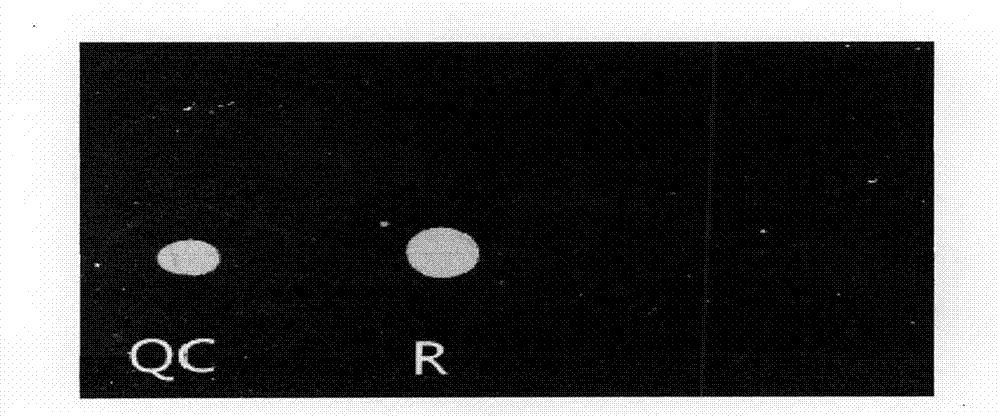
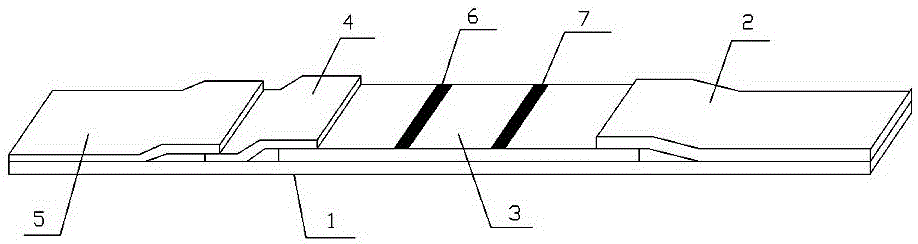
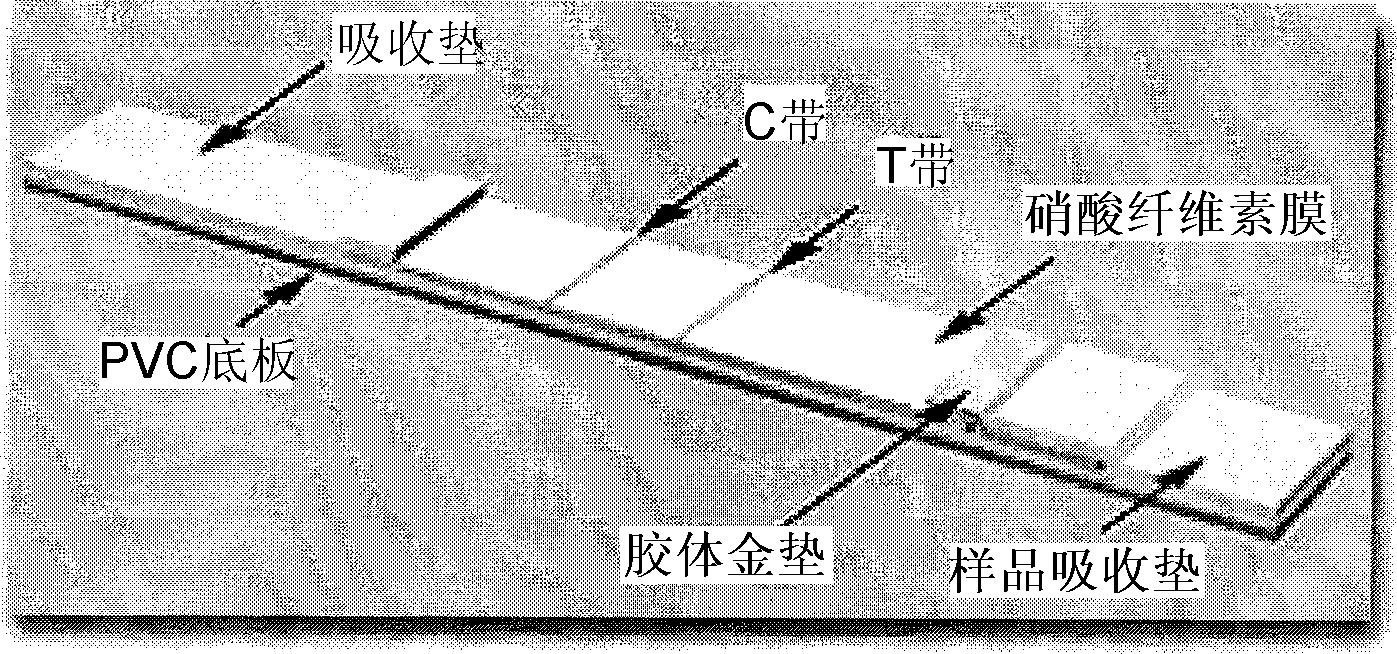
The invention provides a rubella virus immunoglobulin G and immunoglobulin M (IgG and IgM) antibody joint inspection kit and a preparation method thereof. The kit comprises a sample pad (1), a colloidal gold pad (2), a nitrocellulose membrane (3), a sample absorption pad (4) and a bottom plate (5), wherein the colloidal gold pad is a colloidal gold-labeled rubella virus antigen glass fiber (or a non-woven fabric); and the nitrocellulose membrane is coated with a mouse anti-human IgM antibody and a rubella virus antigen serving as detection lines, and goat anti-mouse IgG serving as a quality control line in turn. Rubella virus IgG and IgM antibodies are detected by specific antigen antibody reaction and a colloidal gold immunochromatography technology and can be jointly detected through one-time operation, so that the operation process is simplified, and the kit has the characteristics of quick response, high sensitivity and the like, is easy to operate and is economical and practical.
Owner:北京库尔科技有限公司
Method and kit for detecting RV RNA (Rubella Virus Ribose Nucleic Acid)
ActiveCN103725799AStrong specificityHigh purityMicrobiological testing/measurementMicroorganism based processesLower limitRNA extraction
The invention provides a method for extracting, purifying and detecting RV RNA (Rubella Virus Ribose Nucleic Acid), and a corresponding kit for detecting the RV RNA. The kit comprises a RNA extracting solution containing magnetic beads, and a PCR (Polymerase Chain Reaction) reaction liquid containing an upstream primer, a downstream primer and a probe, wherein the upstream primer and the downstream primer are used for amplifying target polynucleotides; the probe is used for detecting the target polynucleotides. The kit can be used for detecting the RV RNA and cannot be used for detecting non-RV pathogens, thereby illustrating that the kit has the good specificity. In addition, the RNA is extracted by selecting a method of the magnetic beads which are good in adsorption effect and easy to purify, so that the RNA with a high purity and a high yield can be obtained. Thus, the detection sensitivity, the detection accuracy and the detection stability of the kit are greatly improved, wherein the lower limit of detection of the RNA, namely, the sensitivity of the kit can reach 400copies / ml; the detection range of the kit (the quantitative linear range of the kit) can reach 4.00E+02copies / ml to 4.00E+08copies / ml.
Owner:SANSURE (SHANGHAI) GENE TECH LTD
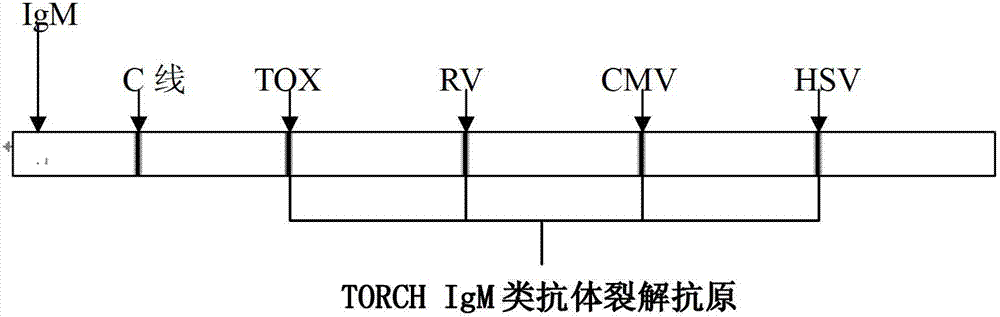
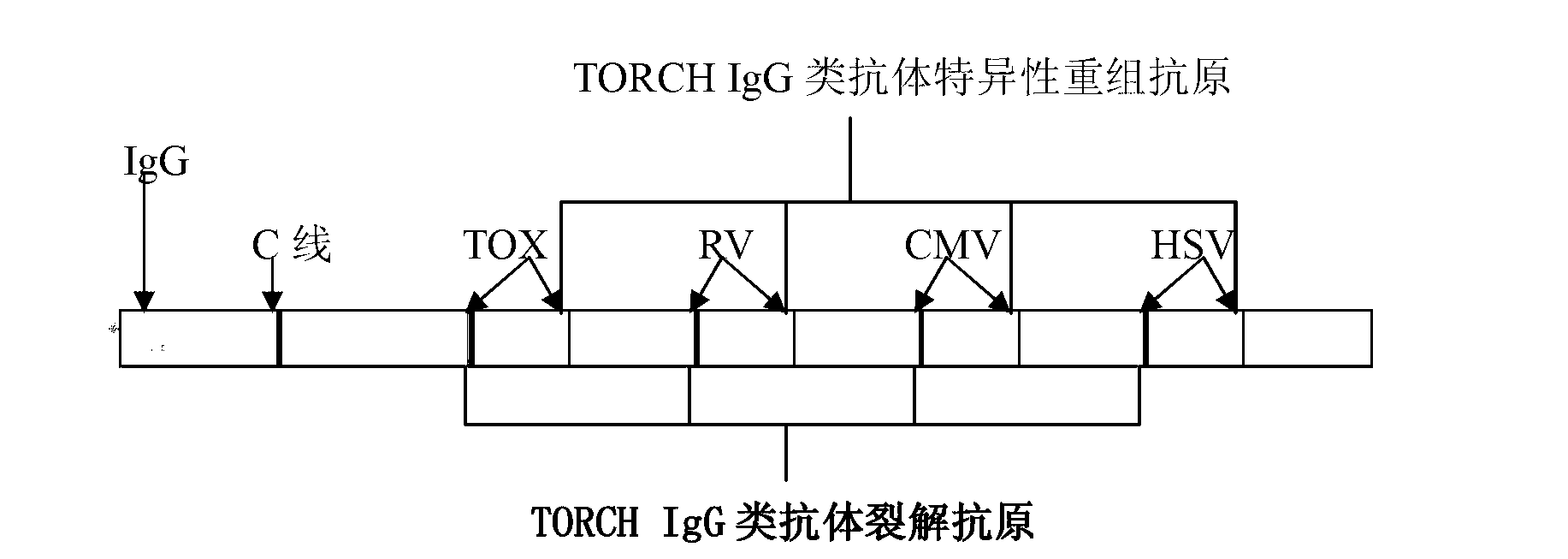
Probe used for TORCH detection, gene chip and kit thereof
ActiveCN104846074AMeet the needs of on-site testingImprove detection efficiencyNucleotide librariesMicrobiological testing/measurementShootFluorescence
The invention discloses a set of probes used for TORCH detection, and comprises a toxoplasma TOX probe which is shown in a SEQ ID NO. 1; a rubella virus RV probe which is shown in a SEQ ID NO.2; a cytomegalovirus CMV probe which is shown in a SEQ ID NO.3; a herpes simplex virus HSV I type probe which is shown in a SEQ ID NO.4; and a herpes simplex virus HSV II type probe which is shown in a SEQ ID NO.5. The invention also comprises a gene chip containing the probe and a kit thereof, visible light passing through a biochip can be amplified, signal resolution is high, and a signal can be shoot by a common digital camera or directly read by naked eyes. Compared with a traditional fluorescent label, the gene chip has good effect. Through specific primer and probe selection, by combining a gene chip technology, detection efficiency and accuracy degree are increased, and requirement of on-site detection for hospital and antenatal testing.
Owner:ZHUHAI SINOCHIPS BIOSCIENCE CO LTD
Preparation method and application of gene chip for detecting important respiratory pathogenic viruses
InactiveCN102586475AStrong specificityImprove the consistency rateMicrobiological testing/measurementOligonucleotide chipMeasles virus IgG
The invention relates to a gene chip for detecting important respiratory pathogenic viruses. A preparation method of the gene chip comprises the following steps of: preparing a specific primer; preparing a virus specific probe; preparing an oligonucleotide chip; establishing an RT-PCR (Reverse Transcription-Polymerase Chain Reaction) system; establishing a hybrid system; preparing a visual detection reagent; and establishing a developing method. The gene chip prepared by the invention can be used for simultaneously discriminating nine general respiratory pathogenic viruses comprising A and B type influenza viruses, parainfluenza viruses type 1 and 2, human metapneumovirus, respiratory syncytial virus, measles virus, rubella virus and mumps virus, provides a new solution for quickly detecting the general respiratory pathogenic viruses at high throughout and can provide guidance for monitoring, clinically diagnosing and treating the respiratory pathogenic viruses.
Owner:深圳市普瑞康生物技术有限公司 +1
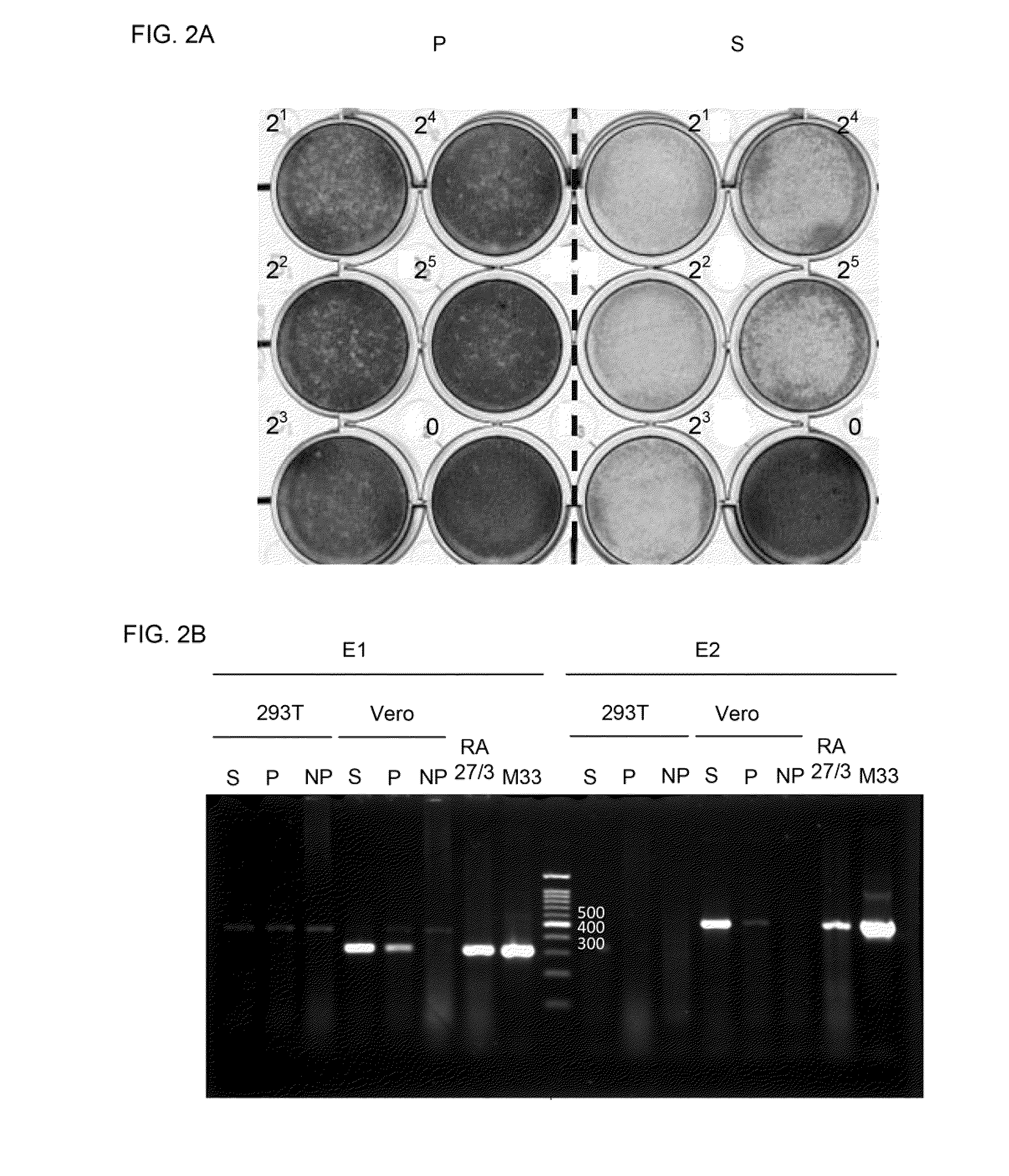
Polyvalent chimeric rubella virus-based vaccines
ActiveUS20100272747A1SsRNA viruses negative-senseSsRNA viruses positive-senseNucleotideNucleotide sequencing
A chimeric viral particle that comprises a RV fusion gene is disclosed. The RV fusion gene comprises a first nucleotide sequence encoding a RV that is devoid of RV E1 protein, and a second nucleotide sequence that linked in translation frame to the first nucleotide sequence and encodes a humoral immunogenic viral protein. The chimeric viral particle is free of RV E1 protein-encoding gene. A virus packaging cell that generates the chimeric viral particle comprising a RV fusion gene and an isolated expression vector comprising a RV fusion gene linked in translation frame to a promoter are also disclosed.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH
Test paper strip for rapidly detecting morbilli and rubella virus IgG antibody colloidal gold
ActiveCN101363856AHigh sensitivityImprove featuresMaterial analysisRubulavirus InfectionsSpecific igg
The invention provides a test strip for simultaneous detection of measles and rubella virus specific IgG antibodies, which comprises a reaction film and a conjugate release pad. The reaction film has a detection band simultaneously coated with measles virus H antigen and rubella virus E1 specific antigen, and a quality control band coated with double-antibody IgG. The conjugate release pad is coated with colloidal gold labeled anti-human IgG. The test strip is simple in operation, convenient, and fast, and has the advantages of no requirements of special instruments and special training, clear and identified result, and easy popularization. The test strip is suitable for base and site detection and epidemiological investigation, has auxiliary and differential diagnosis effects on measles and rubella virus infection, and can be used for the immune effect observation after vaccination.
Owner:辽宁迪浩生物科技有限公司
Preparation method and application of fluorescent marker gene chip reagent for detecting ToRCH (toxopasma, rubella virus, cytomegalo virus and herpes virus)
InactiveCN103805715AHigh sensitivityShort time consumptionMicrobiological testing/measurementFluorescenceTorch
The invention discloses a preparation method of a fluorescent marker gene chip reagent which can be used for simultaneously detecting ToRCH, namely five pathogens such as toxopasma, rubella virus, cytomegalo virus and pure herpes virus type I / II. Compared with the prior art, the preparation method has the characteristics of high specificity, high sensitivity and short operation time, and therefore, the preparation method has broad application prospect. In addition, the invention provides the reagent obtained by the preparation method and a kit used for the detection method.
Owner:TAIYUAN UNIV OF TECH
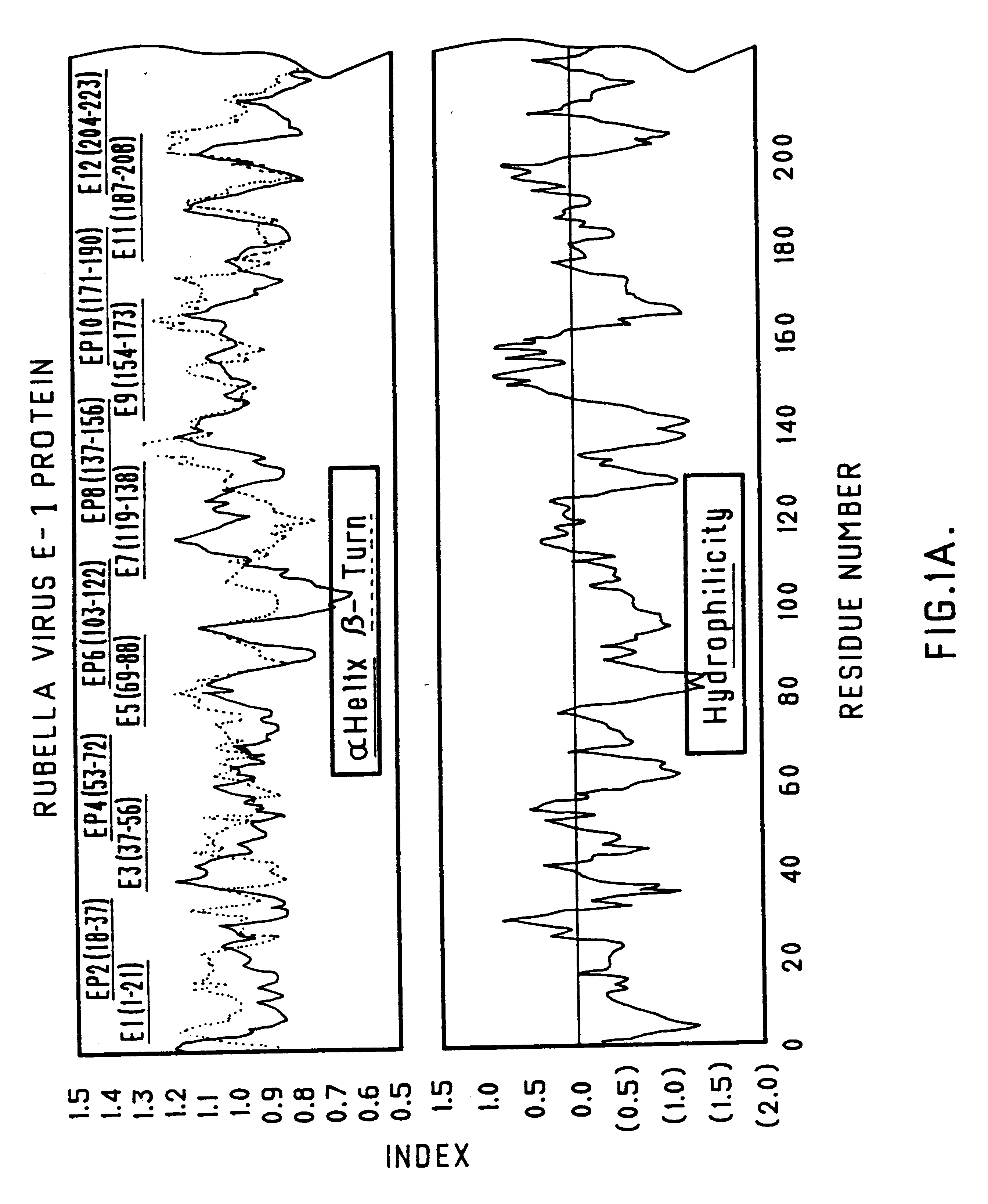
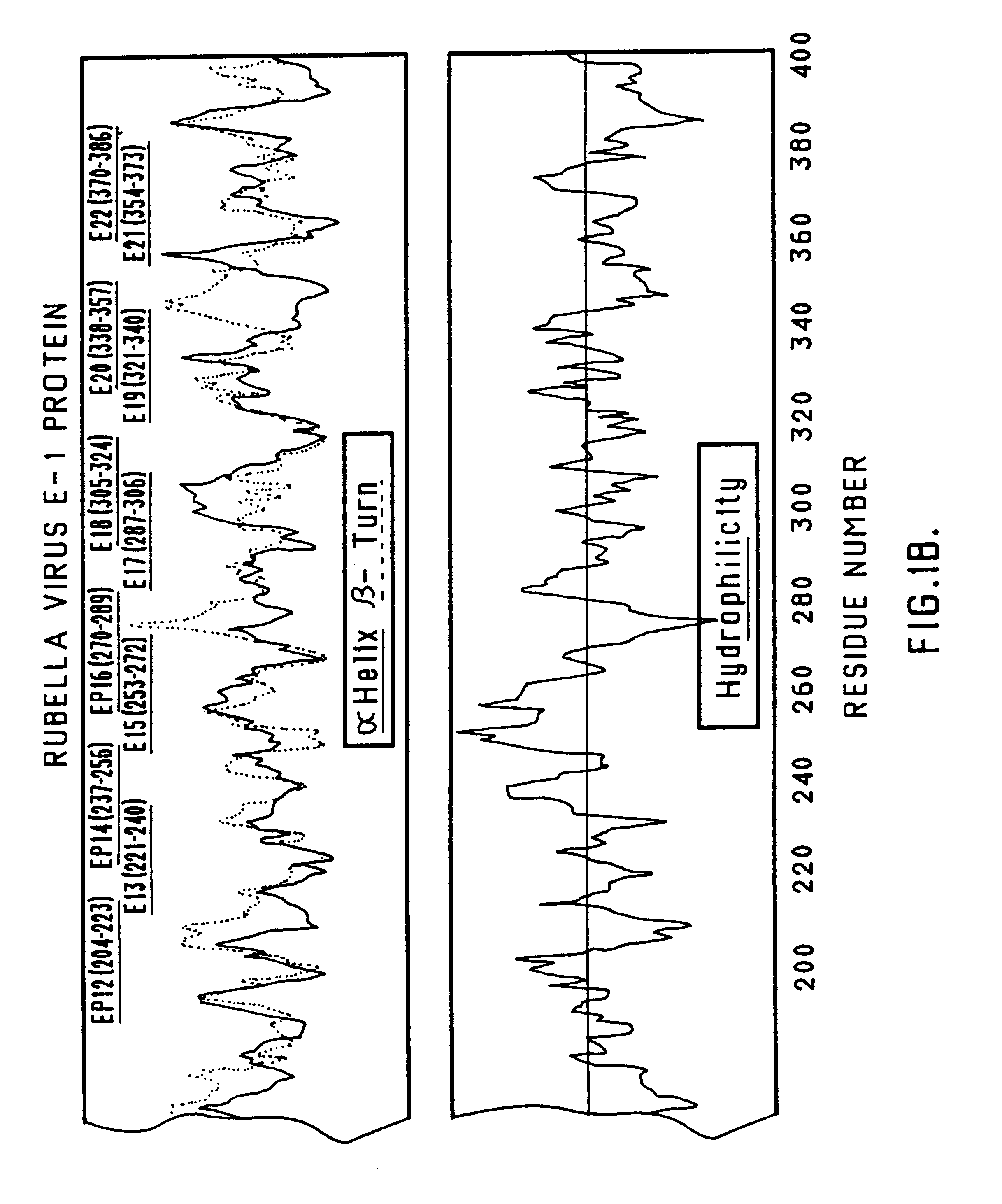
Recombinant rubella virus E1 protein and uses thereof
InactiveCN101402960AMeet the needs of clinical diagnosis of infectionStrong specificityBacteriaImmunoglobulins against virusesRubulavirus InfectionsIgm antibody
The invention relates to a recombinant rubella virus E1 protein and an application thereof. The adopted technical proposal is as follows: the amino acid sequence of the recombinant rubella virus E1 is shown in SEQ ID NO: 2, a reagent kit which is prepared by the recombinant rubella virus E1 protein and used for detecting the rubella virus IgM antibody infection is adopted, compared with the similar reagent kits on the market, the reagent kit has the advantages of strong specificity, high sensitivity, and the like, thereby well meeting the needs of clinical diagnosis of rubella virus infection. The invention further provides the application of the recombinant rubella virus E1 protein in the preparation of monoclonal antibodies, polyclonal antibodies and protein chips.
Owner:吴丽霞 +2
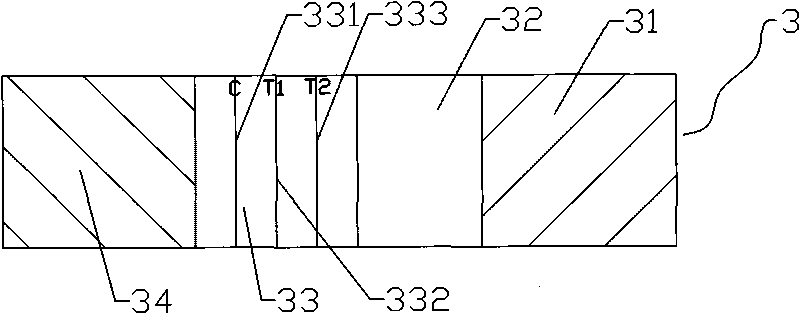
Combined test reagent card for cytomegalovirus and rubella virus
ActiveCN101738474AHigh compliance rateImprove accuracyMaterial analysisNitrocelluloseCytomegalovirus antigen
The invention discloses a combined test reagent card for cytomegalovirus and rubella virus, which comprises a card cover, a card bottom and a test strip. The test strip is provided with a test sample region, a gold label region, a nitrocellulose membrane region and a water absorption region sequentially; and the nitrocellulose membrane region is provided with a quality-controlling line coated with goat anti mouse IgG, a testing line coated with cytomegalovirus antigen and a testing line coated with rubella virus antigen sequentially. The technical scheme adopted by the invention can test cytomegalovirus IgG antibody and rubella virus IgG antibody in the sample simultaneously and the total accordance rate of the testing result is higher, so the application prospect is wide. Compared with the detection of IgM antibody, the combined test reagent card for the cytomegalovirus and the rubella virus tests the IgG antibody; the IgG antibody can be carried for the whole life and is only associated with acute infection; and the accuracy is high.
Owner:山东康华生物医疗科技股份有限公司
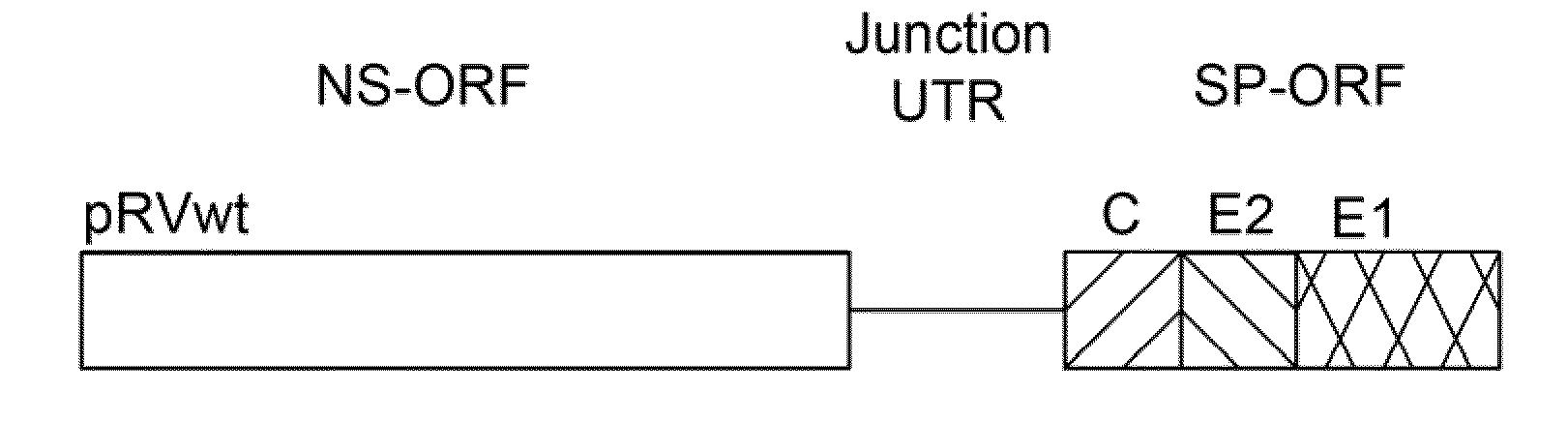
Highly infectious rubella virus DNA constructs and methods of production
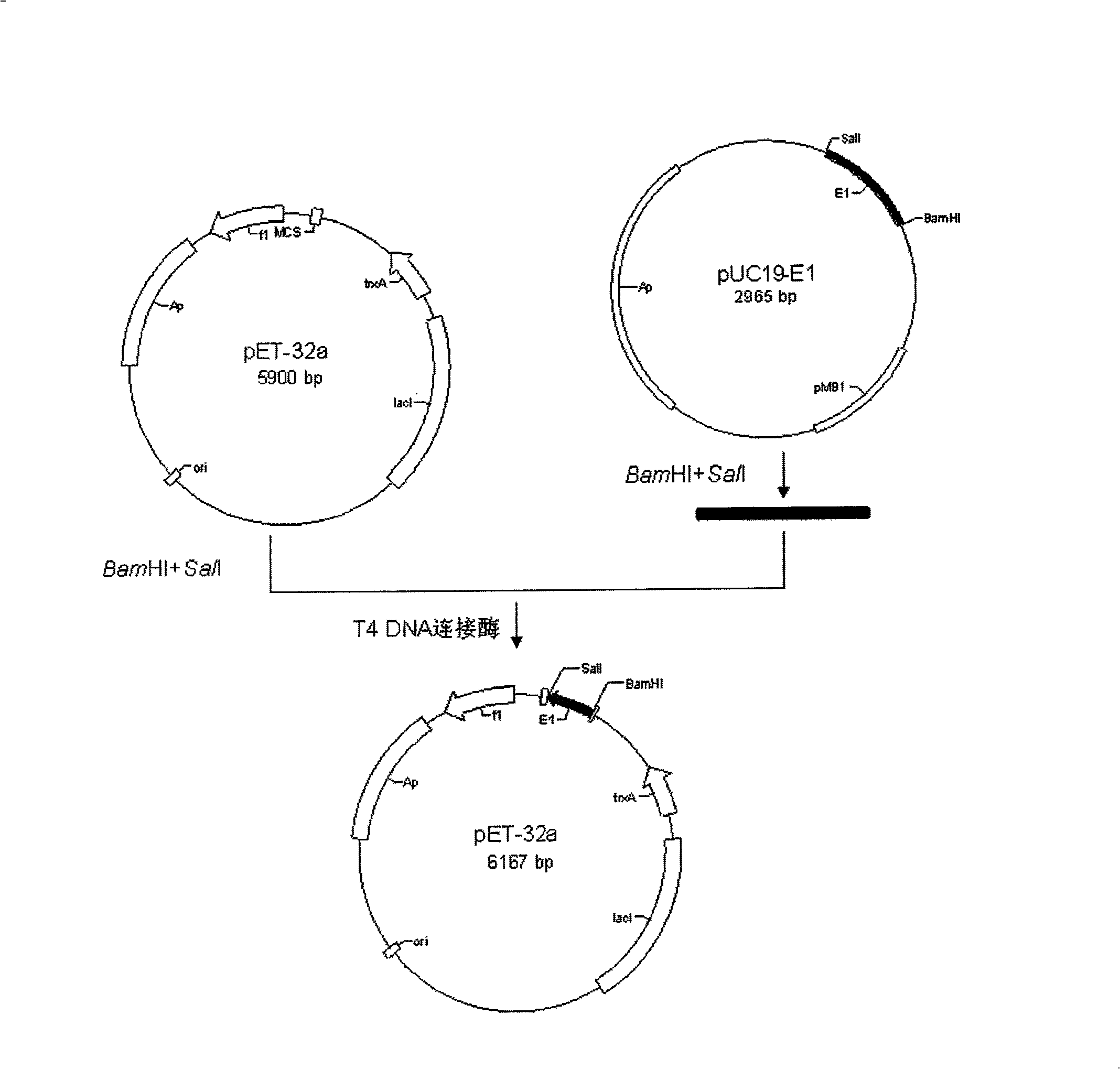
InactiveUS6958237B2Improve stabilityHigh expressionSsRNA viruses positive-senseVectorsOpen reading frameDNA construct
Highly infectious rubella virus cDNA clones derived from infectious cDNA clone having a low specific infectivity and methods of obtaining highly infectious rubella virus cDNA clones. Togavirus expression vectors of improved stability for the expression of live, attenuated togavirus and a foreign gene, based on the nucleic acid sequence of an infectious rubella virus clone and contain a togavirus non-structural protein open reading frame; an expression element for expression of a foreign gene; a foreign gene or a multiple cloning site for insertion of a foreign gene; an expression element for the expression of the live, attenuated togavirus; and a togavirus structural protein open reading frame. The expression element is either a subgenomic promoter or an internal ribosome entry site (IRES). Administration of the vector as an immunization agents is useful for the induction of immuity against the togavirus, the foreign gene, or both.
Owner:GEORGIA STATE UNIV RES FOUND INC
Primer probe combination for specific detection of measles virus and rubella virus and kit
ActiveCN102634610ALow detection costStrong specificityMicrobiological testing/measurementMicroorganism based processesDisease monitoringSpecific detection
The invention relates to an oligonucleotide sequence combination for specific detection of nucleic acid of measles virus and rubella virus in a sample by utilizing a fluorescent PCR (polymerase chain reaction) technology, as well as a kit including the combination. The kit can sensitively detect and identify the nucleic acid of the measles virus and the rubella virus in the sample, the detection lower limit is 20 copies per reaction system, and the kit further has an important application value in the fields of disease monitoring, clinical diagnosis and the like.
Owner:JIANGSU UNINOVO BIOLOGICAL TECH
Method for preparing ToRCH-ELISA (Enzyme Linked Immunosorbent Assay Kit) diagnosis reagent
The invention discloses a method for preparing a ToRCH-ELISA (Enzyme Linked Immunosorbent Assay Kit) diagnosis reagent. The method for preparing the ToRCH-ELISA diagnosis reagent comprises the following steps: preparing a rubella virus, herpes simplex viruses I and II, a cytomegalovirus and toxoplasm antigen, and establishing an ELISA. The (Enzyme Linked Immunosorbent Assay Kit) diagnosis reagent is prepared by preparing the rubella virus, the herpes simplex viruses I and II, the cytomegalovirus and the toxoplasm antigen and establishing the ELISA. The method has the technical effects of rapidness, sensitivity, high specificity and good repeatability.
Owner:严银芳
Anti-human IgM monoclonal antibody, hybridoma cell strain and application thereof
ActiveCN108517315AImprove immunityGood specific binding abilityMicroorganism based processesTissue cultureRubulavirus InfectionsHybridoma cell
The invention relates to a hybridoma cell strain and a secreted monoclonal antibody thereof; the antibody can be specifically combined with a human IgM, and can be used for in vitro detection of the human IgM, and particularly suitable for early diagnosis of rubella virus infection. The invention further relates to a kit comprising the hybridoma cell strain or the monoclonal antibody.
Owner:SICHUAN ANKERUI NEW MATERIAL TECH CO LTD
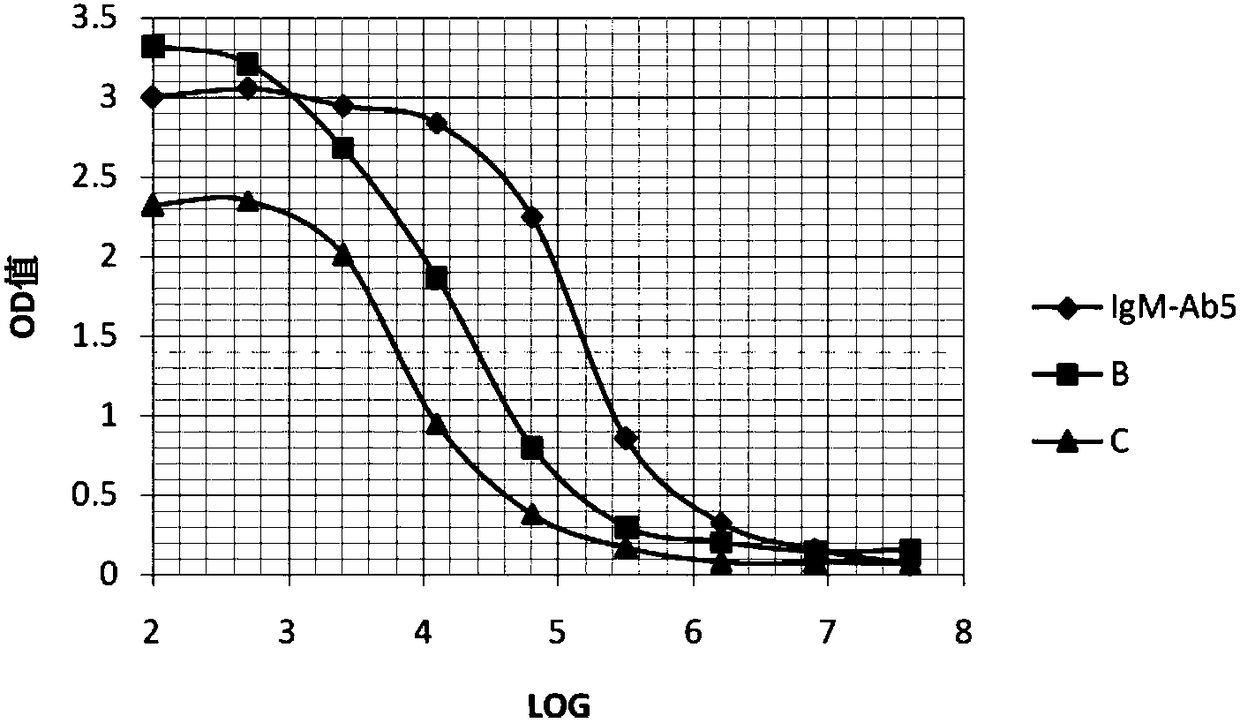
IgM antibody detection kit for five TORCH tests and preparation of IgM antibody detection kit
InactiveCN103033612ASample requirement is smallStrong specificityMaterial analysisPathogenic microorganismIgm antibody
The invention relates to a pathogenic microorganism detection kit, in particular to an IgM antibody detection kit for five TORCH tests and preparation of the IgM antibody detection kit. The kit is used for detecting IgG-class antibodies in a detection sample in five TORCH tests, namely TOX, rubella virus (RV), cytomegalovirus (CMV) and herpes simplex I / II virus (HSV-I / II).
Owner:BEIJING KINGHAWK PHARMA
Synthetic peptides for rubella vaccine
Synthetic peptides have an amino acids sequence corresponding to at least one antigenic determinant of at least one protein, usually a structural protein, particularly the E1, E2 or C proteins, of rubella virus (RV), are used as is, in hybrid or chimeric tandem T-B form, in lipidated form, linked to a carrier molecule and / or polymerized to form molecular aggregates, in vaccines against rubella. Analogs of peptides which are human T-cell determinants are used to treat rubella-associated autoimmune disorders.
Owner:CONNAUGHT LAB
Nucleic acid detection kit for quickly detecting measles virus/rubella virus
ActiveCN103409554ASimple and fast operationAvoid pollutionMicrobiological testing/measurementMicroorganism based processesPositive controlDisease surveillance
The invention discloses a nucleic acid detection kit for quickly detecting a measles virus / a rubella virus. The kit comprises an RT-PCR (Reverse Transcription-Polymerase Chain Reaction) reaction liquid, an enzyme mixed liquid, a measles virus / rubella virus reaction liquid, positive control and negative control. The kit disclosed by the invention overcomes the deficiencies that the prior art, almost measles virus / rubella virus detection kits are poor in specificity, relatively low in sensitivity and the like, has the advantages of high throughput, strong repeatability, quick and objective detection result and the like, is simple and convenient to operate and has huge application prospect in the fields such as clinical diagnosis and disease surveillance.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
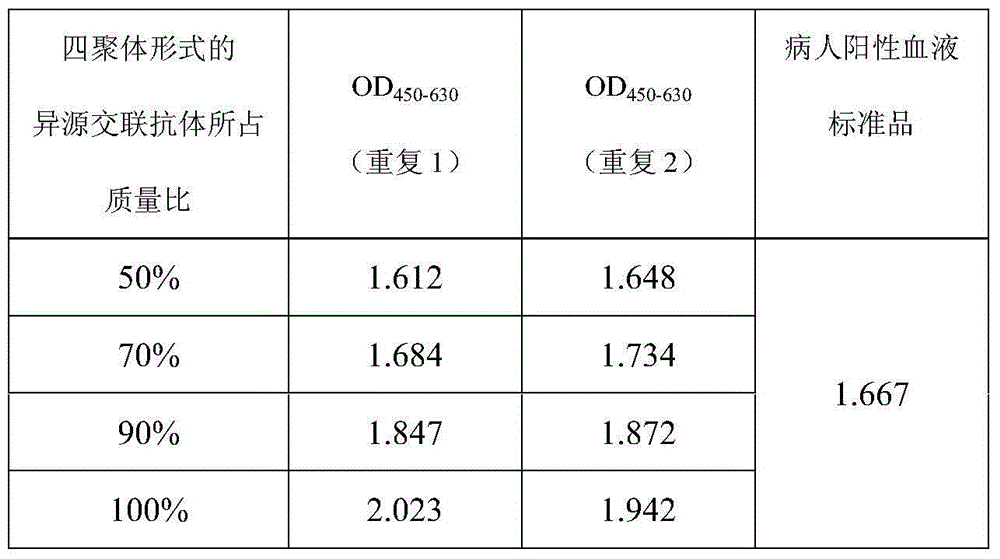
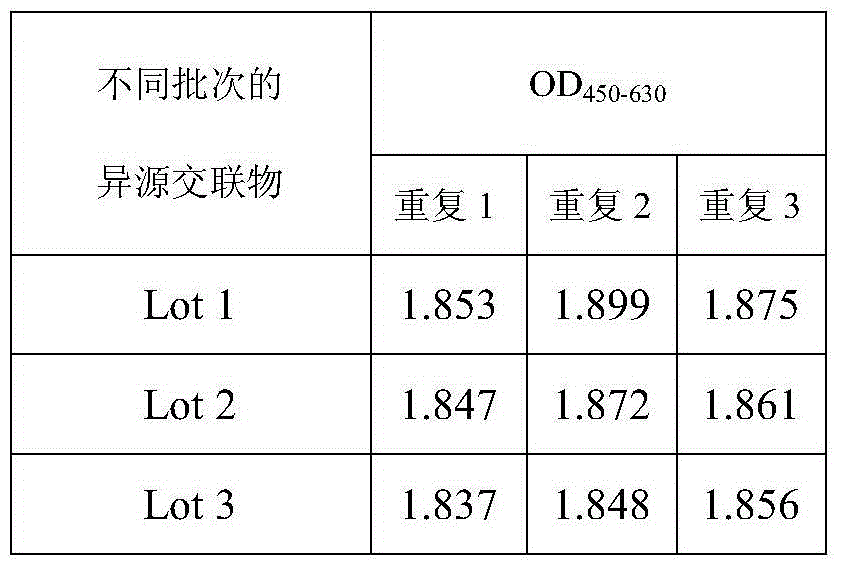
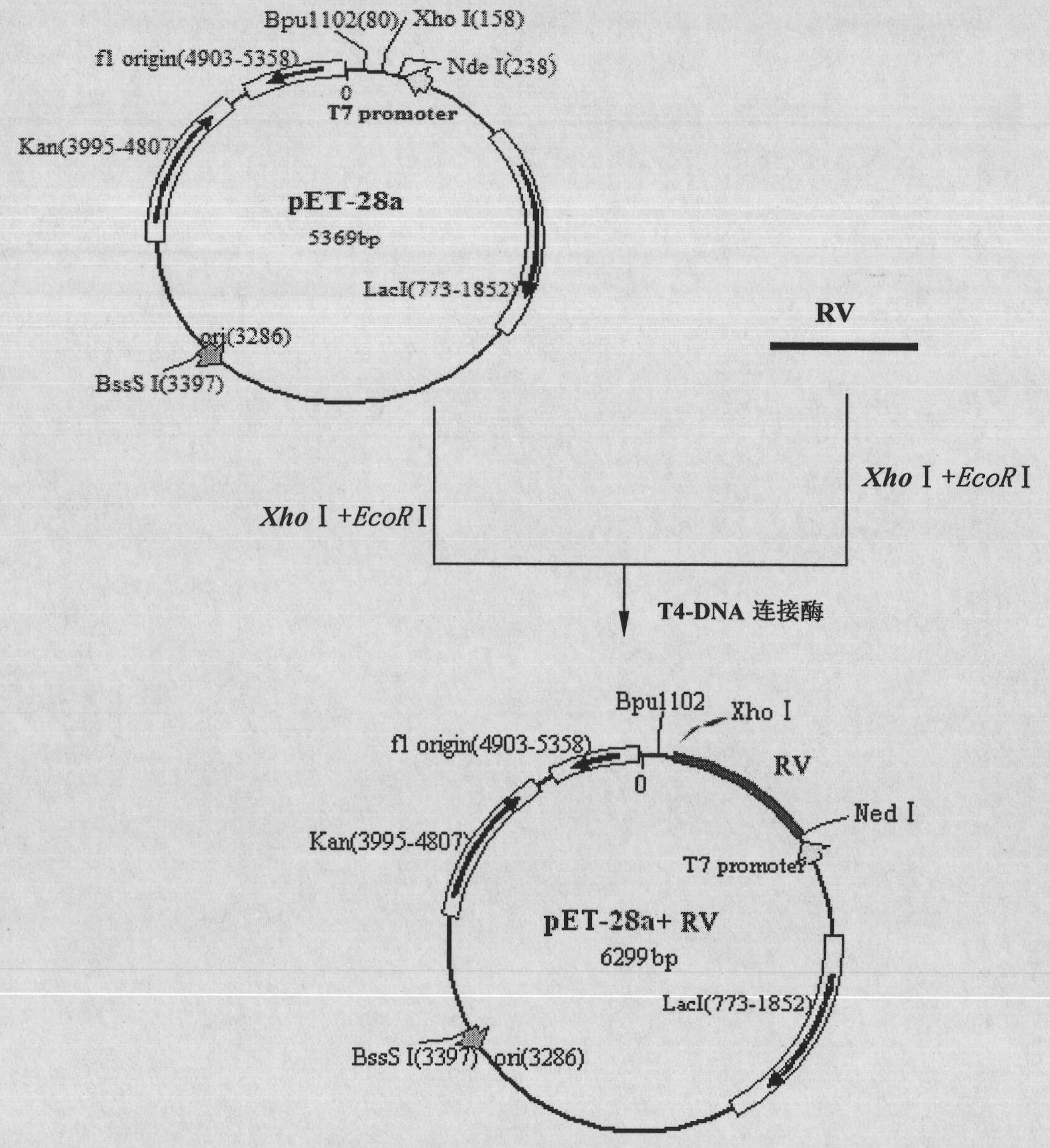
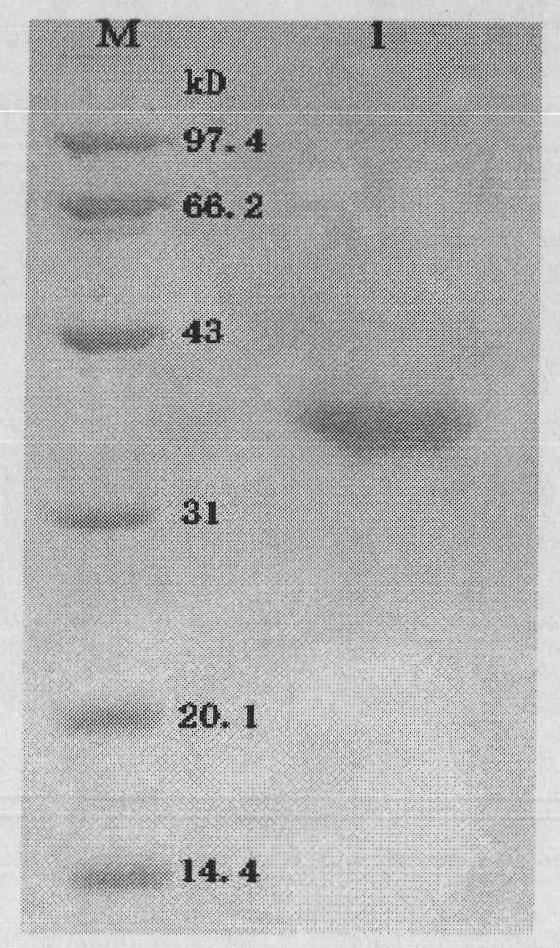
Heterogenous conjugate and application thereof to RV (rubella virus) assay
ActiveCN104650236AEasy to produceLow costHybrid immunoglobulinsMaterial analysisHeterologousRubella virus antibody
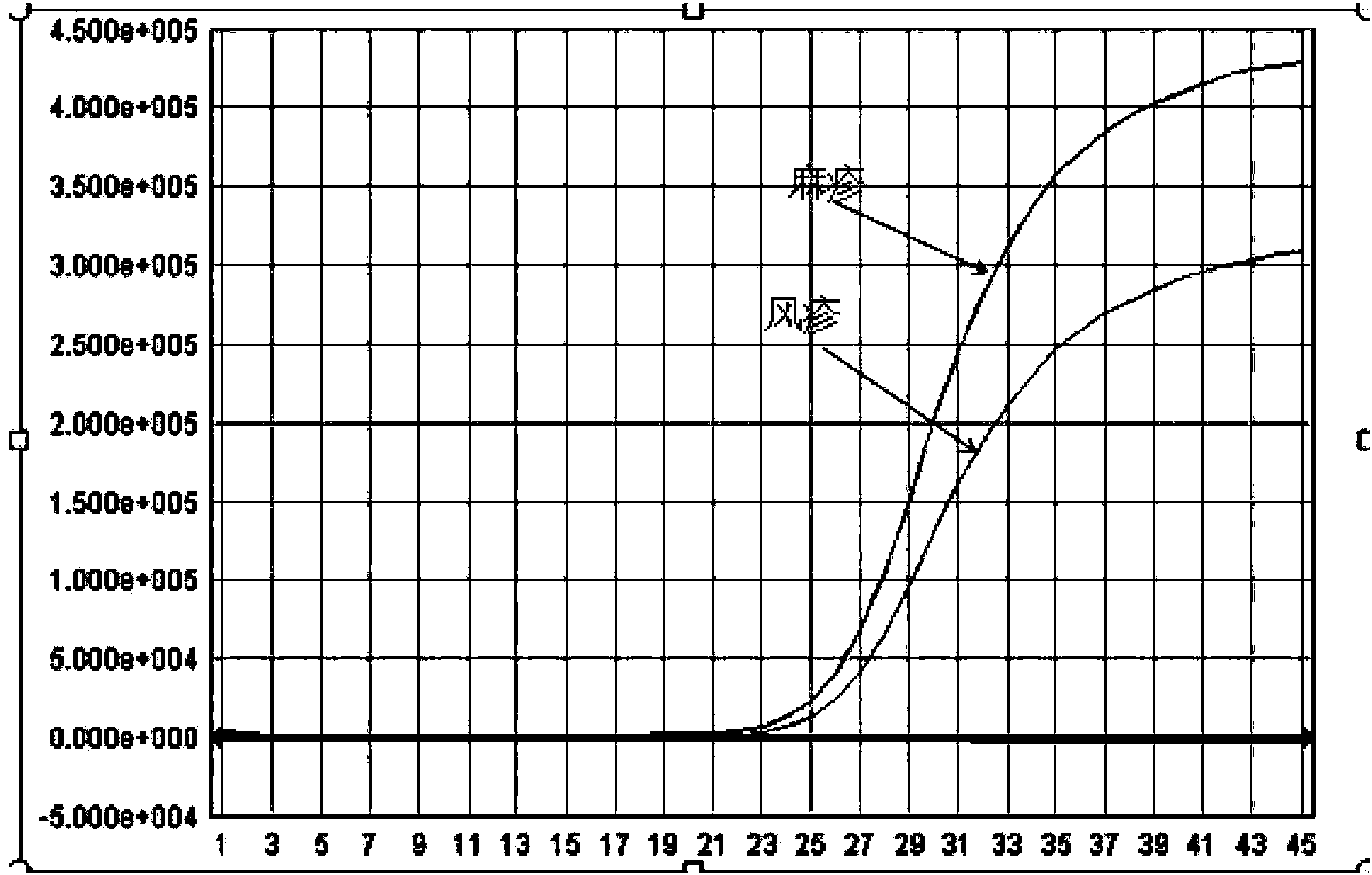
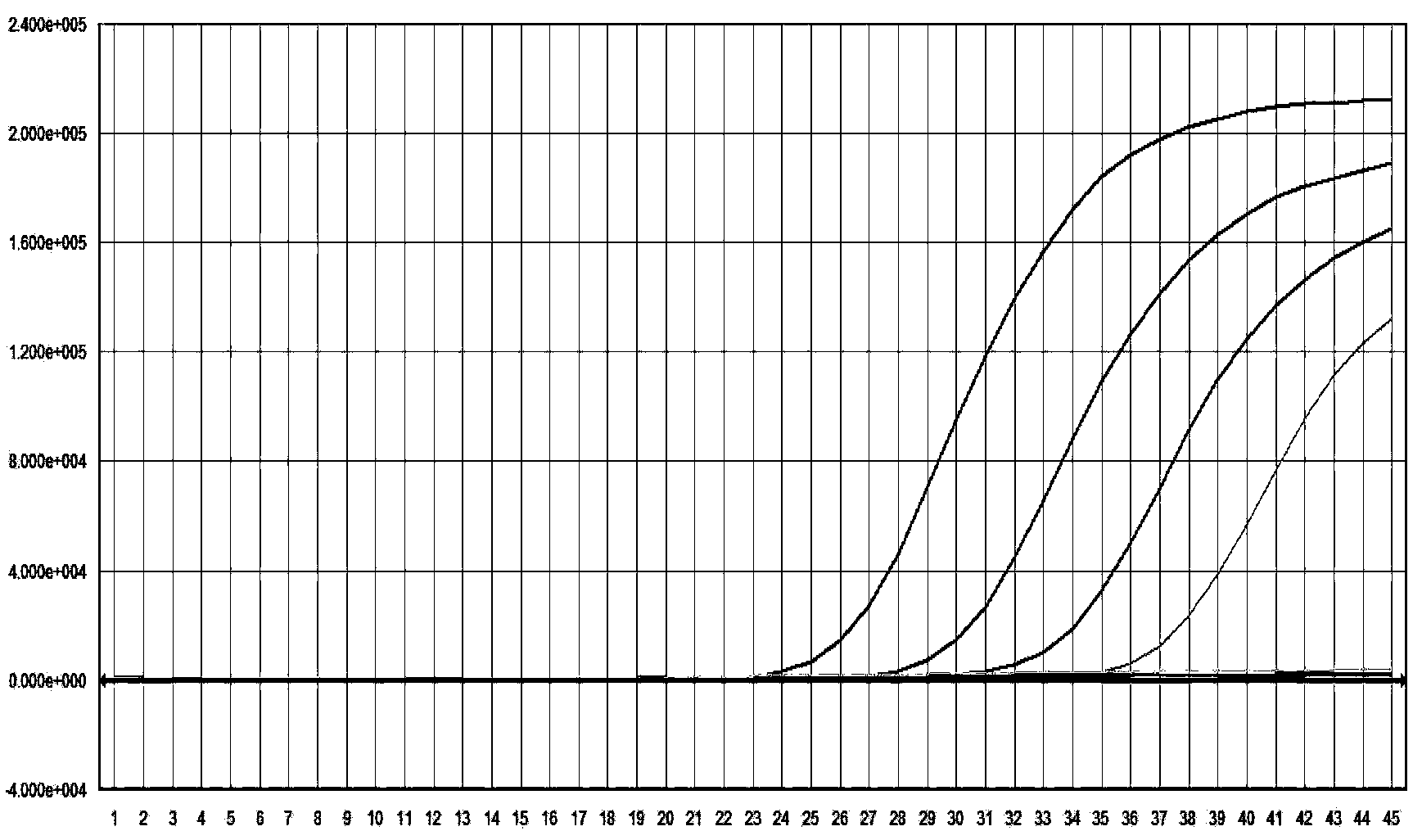
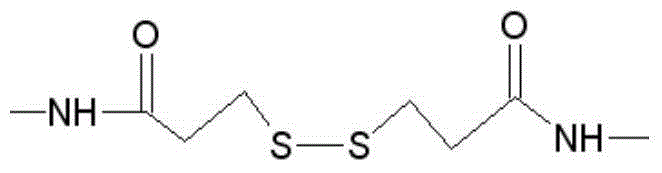
The invention provides a heterogenous conjugate. The heterogenous conjugate comprises a tetramer shaped heterogenous crosslinking antibody formed by mouse anti-RV (rubella virus) McAb and healthy human IgG (immunoglobulin G) which is not infected with RV, wherein the antibody can serve as a substitute of an RV antibody in the positive blood of patients and can be used in immunoassay kits as a quality control product replacing the positive blood of patients after being diluted in a diluent. The heterogenous conjugate is simple to produce and prepare, is low in cost, has very good safety and stability at the same time and can conduce to reducing the assay errors.
Owner:GENCLONN BIOTECH HANGZHOU
Use of 17-ketosteroid compounds, as well as derivatives, metabolites and precursors for treatment of hapatitis C type virus and other togavirus infections
The present invention provides 17-keto steroid compounds and their derivatives, metabolites and precursors and pharmaceutically acceptable salts thereof for the treatment or prevention of hepatitis C virus and / or hepatitis G virus in patients requiring such treatment, these compounds Collectively referred to as "compounds of the present invention". In addition, the present invention provides methods of treating or preventing togavirus infections including one or more of alphaviruses, flaviviruses (such as yellow fever virus), hepatitis C virus, and hepatitis G virus, Infection with rubella virus or pestiviruses (such as bovine viral diarrhea virus). In addition, the present invention provides combination therapy comprising the administration of one or more compounds described herein and the administration of one or more compounds selected from the group consisting of plasma concentration-enhancing compounds, macrophage stimulating factors, oxidative agents, ribavirin, and alpha interferon compounds and / or oxygen supply. The compounds of the invention may also be used to alleviate or alleviate one or more symptoms associated with togavirus infection.
Owner:HOLLIS EDEN PHARMA
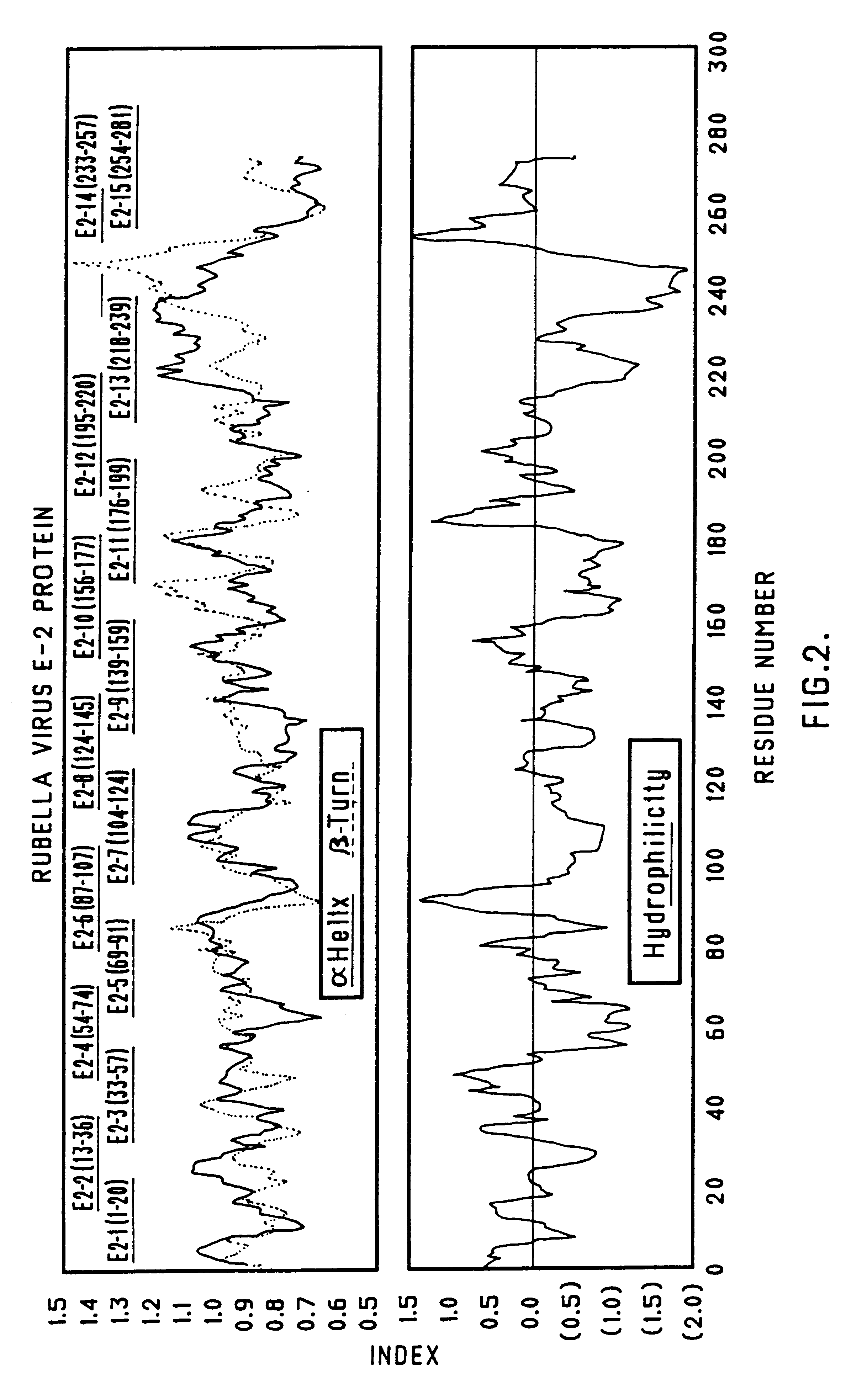
Recombinant rubella virus protein and application
InactiveCN101781360AStrong specificityHigh sensitivityAntiviralsDepsipeptidesEpitopeRubulavirus Infections
The invention discloses a recombinant rubella virus protein, which comprises dominant epitopes E1 fragment and C fragment of the rubella virus protein RV, and is used as a rubella virus antibod IgG immune detection kit for preparing antigen. The invention has the advantages of strong pecificity, high sensitivity and the like compared with the similar kit on market, and better meets the requirements of clinical diagnosis of rubella virus infection. The antigen has high pecificity, strong immunogenicity and complete antigenic determinants as much as possible, and the invention has practical application value in the field of RV vaccine development.
Owner:JILIN UNIV
Rubella virus fluorescence quantitative polymerase chain reaction (PCR) kit and detection method thereof
InactiveCN102061341AStrong specificityHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceRubulavirus InfectionsRNA extraction
The invention discloses a Rubella virus (RV) detection kit and an application thereof. The kit contains RNA extraction solution, reverse transcriptase (RT), RNA enzyme inhibitor, a standard positive template, Taq DNA polymerase, fluorescence quantitative polymerase chain reaction (PCR) reaction solution and a standard negative quality control product. The detection method comprises the following steps: the kit is used to extract the virus DNAs of a sample to be detected, then the virus DNAs along with the standard positive quality control product and the standard negative quality control product perform fluorescence quantitative RT-PCR, and the software of the fluorescence quantitative PCR instrument is utilized to calculate the initial concentration of the RV in the sample. The invention has the advantages of high detection speed, and short time and high efficiency, and is convenient and safe to operate, thus the virus RNA detection can be effectively performed to the patient with RV infection and the early diagnosis and effective prevention of RV infection can be realized.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
Portable fast joint inspection device for foetus teratogenic disease
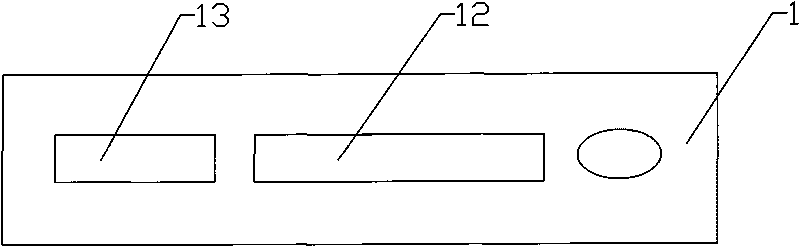
ActiveCN101221177AIndicator detection is simpleIndicator Detection SecurityMaterial analysisDiseaseEngineering
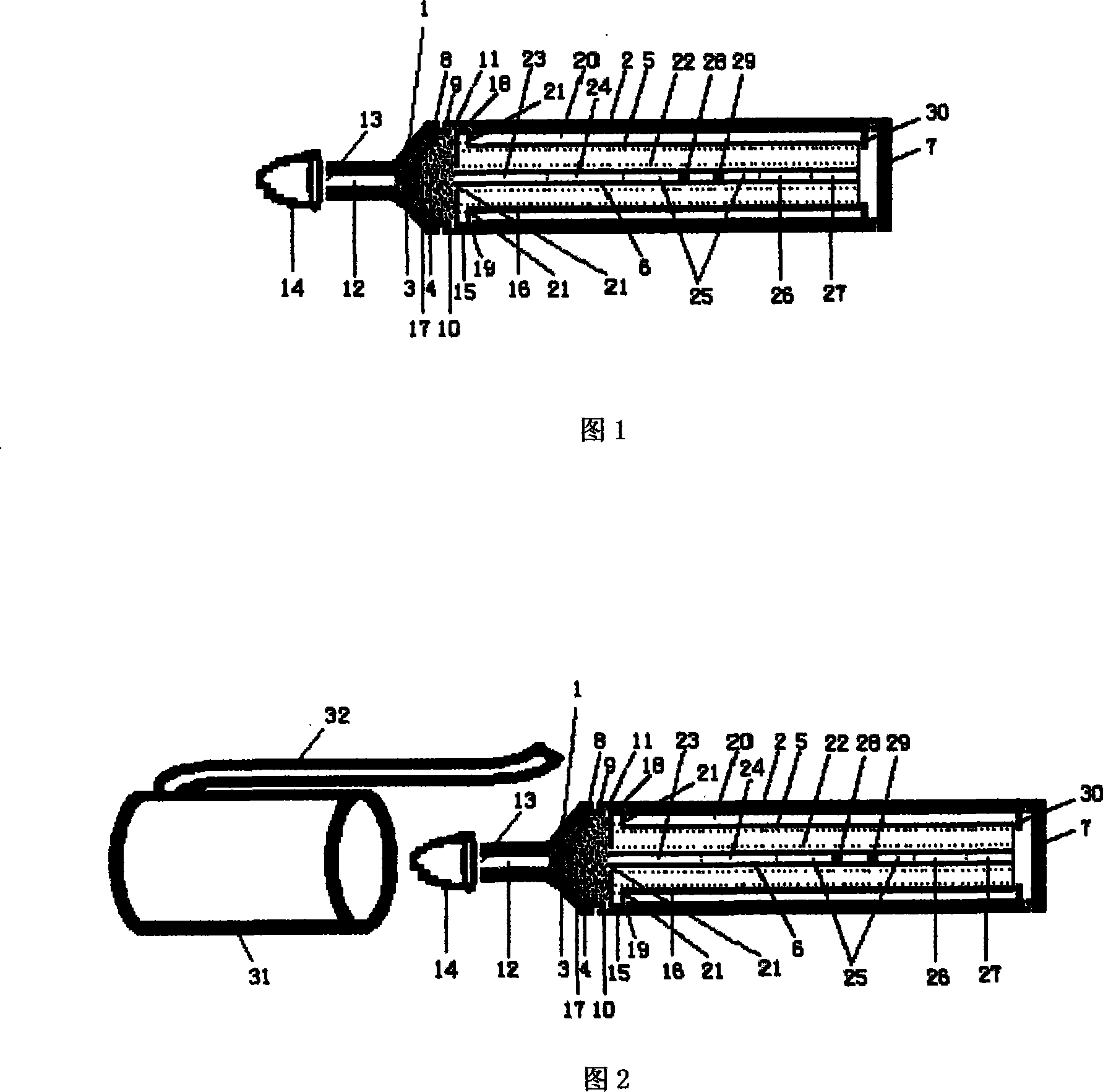
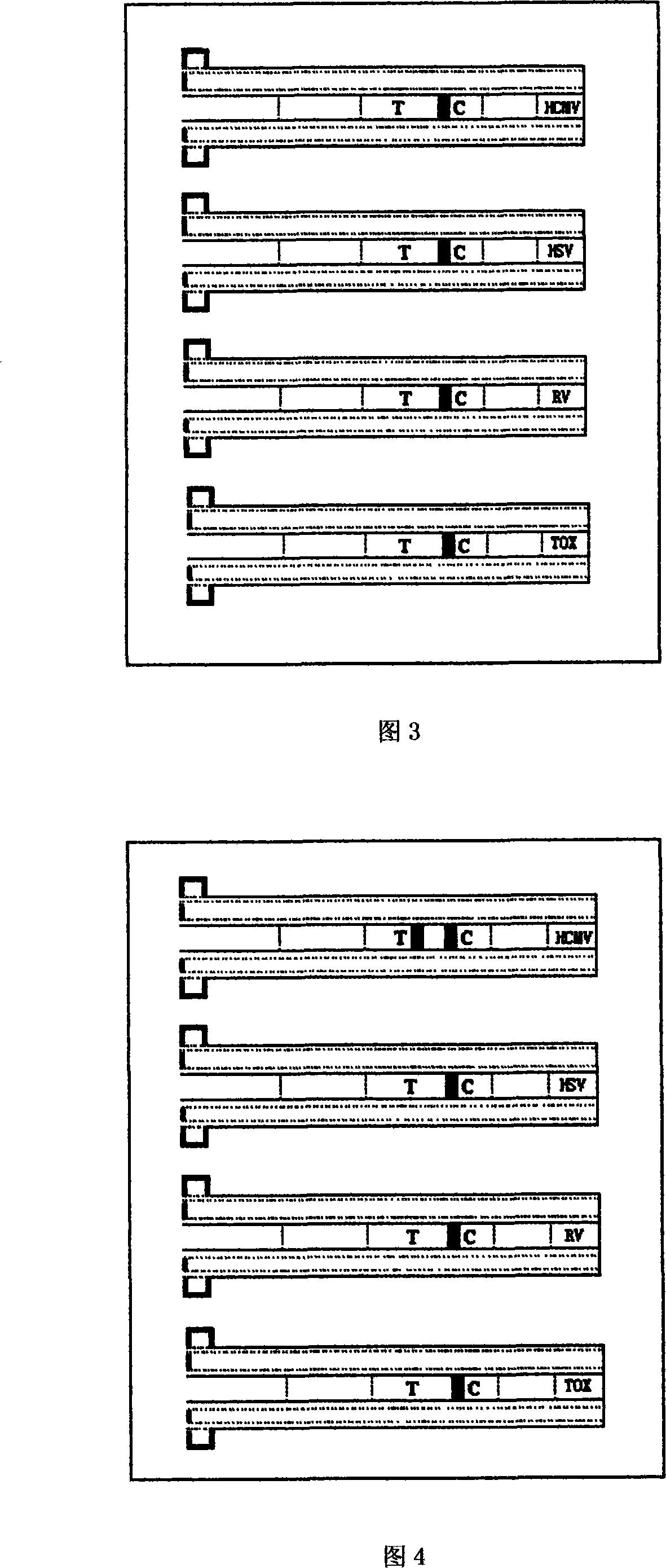
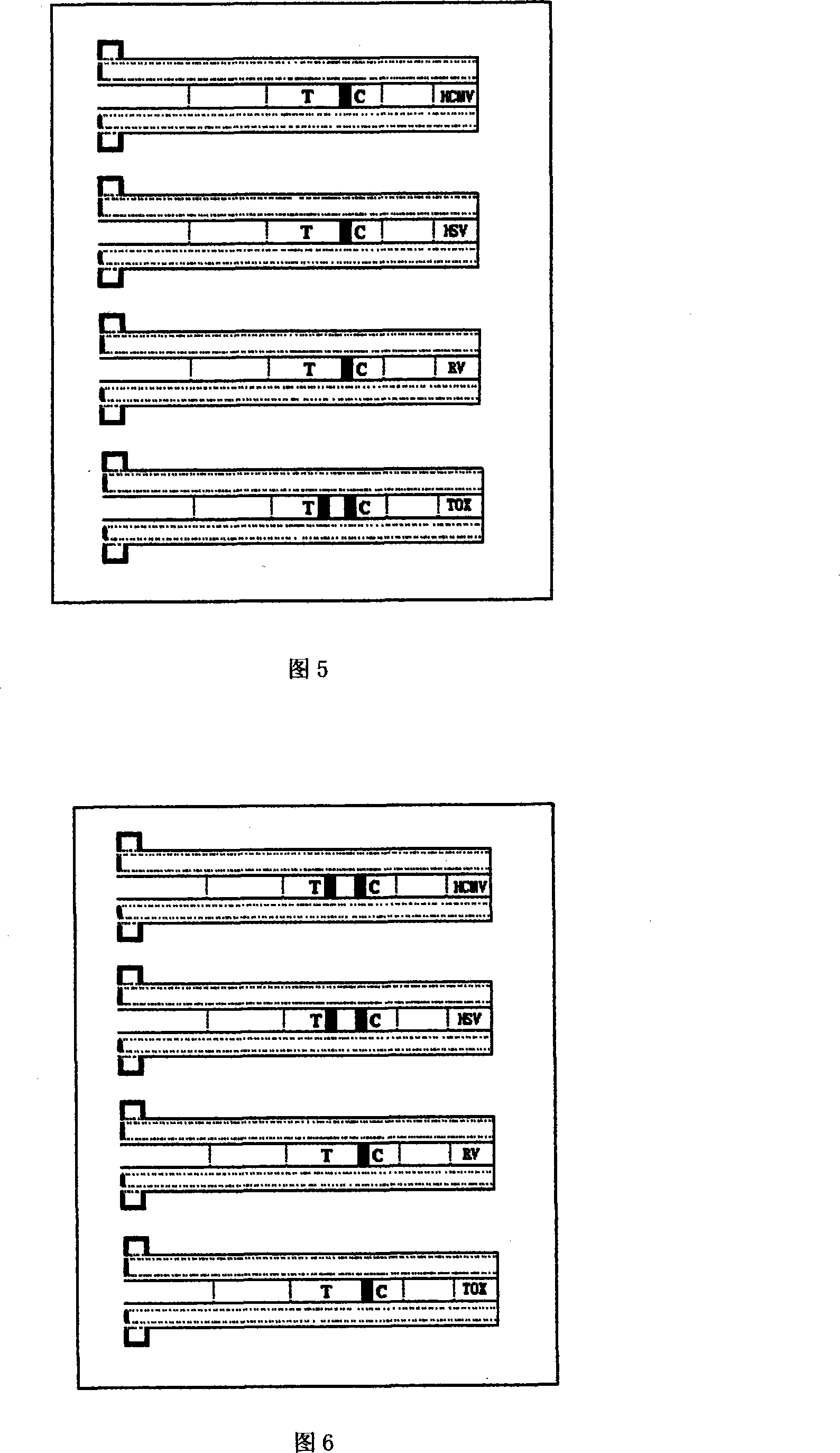
The invention discloses a portable rapid combined detection device of fetal teratogenic diseases, including a sample suction head (1) and a detection tube (2) which is connected at the back end of the sample suction head (1). The front end of the sample suction head (1) is provided with a thin tube suction mouth (12), the suction mouth (12) is provided with a sample suction port (13) for sample suction, the front end of the suction mouth (12) can be sheathed with a rubber protecting cap (14), an inner cavity (17) of the sample suction head can be respectively filled with sample filter materials (3) and sample water absorbent materials (4) from the front to the back, the interior of the detection tube (2) is provided with a test paper fixed column (5), a cytomegalovirus (HCMV) detection test paper strip, a herpes simplex virus (HSV) detection test paper strip, a rubella virus (RV) detection test paper strip and a toxoplasma (TOX) detection test paper strip are fixedly arranged on the test paper fixed column (5), and the tail end of the detection tube is provided with a sealing cover (7) to seal the waste after detection in the detection tube (2), so as to prevent leakage. All the detection test paper strips (6) which are sealed and fixed in the device are carried out one-time sample suction and the detection with same background, the invention can carry out the combined detection of HCMV antibody, HSV antibody, RV antibody and TOX antibody in the sample at one time within a plurality of minutes by using the naked eye, a plurality of detections of the sample become more simple, safe and rapid; furthermore, the cross-interference can be avoided, the waste after detection is still sealed in the detection tube, so the invention is conductive to the avoidance of spread of pathogens and iatrogenic infection, and is more conductive to the environmentally protective treatment of the waste.
Owner:WUHAN J H BIO TECH
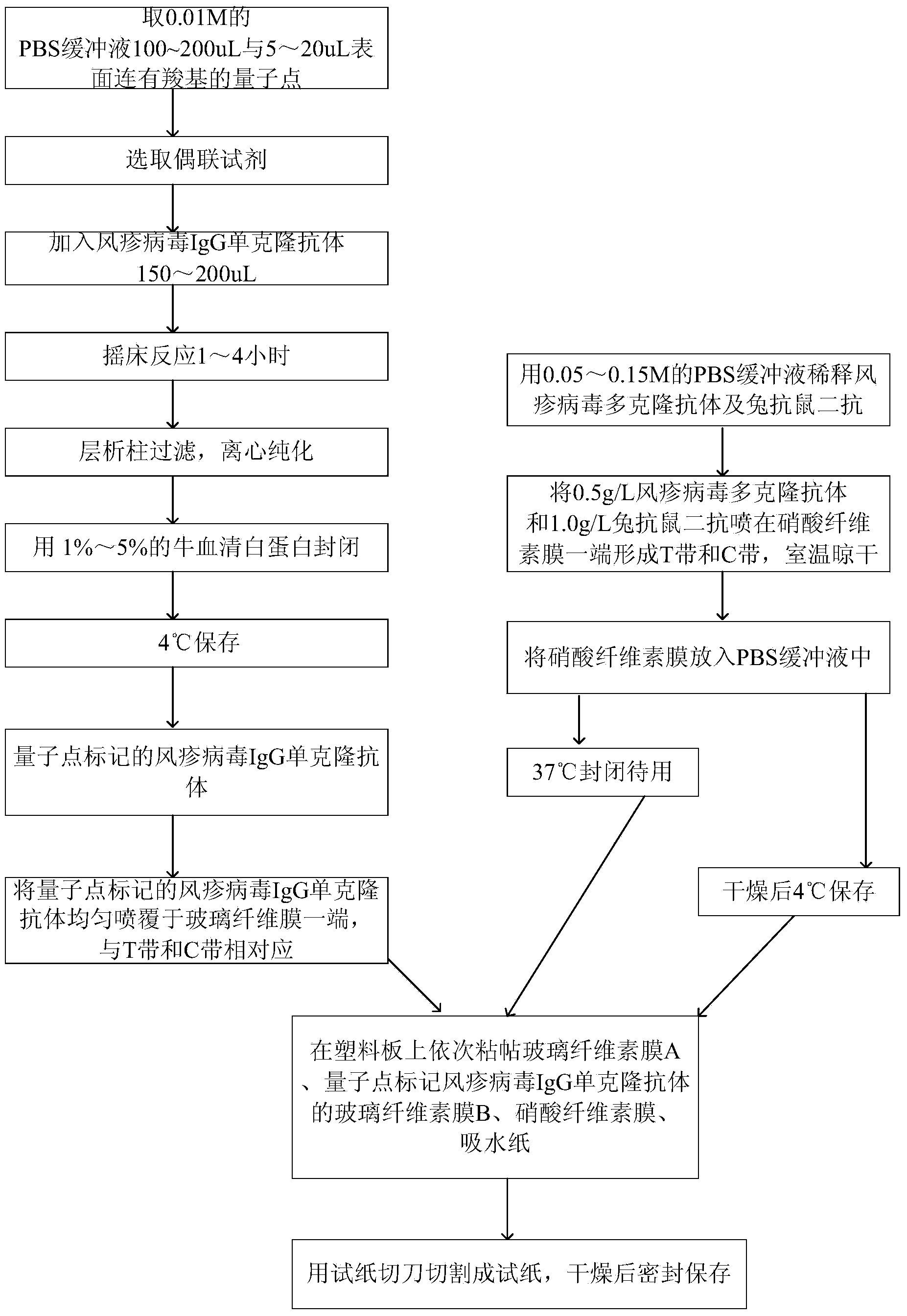
Method for detecting rubella virus, quantum dot-labeled immunochromatographic test paper and preparation method thereof
ActiveCN103529211AHigh detection sensitivityHigh detection sensitivity than a rapid detection method commonly used at present - the detection sensitivity of colloidal goldBiological material analysisCelluloseGlass fiber
The invention relates to a medimmune inspection method, and particularly relates to quantum dot-labeled immunochromatographic test paper, and a method for detecting rubella virus by adopting an immunological method. According to the quantum dot-labeled immunochromatographic test paper, a glass cellulose membrane A, a quantum dot-labeled rubella virus IgG monoclonal antibody glass cellulose membrane B, a cellulose nitrate membrane and absorbent paper are sequentially bonded on a plastic board from bottom to top, wherein a rubella virus polyclonal antibody and a rabbit-anti-mouse secondary antibody are arranged on one end of the cellulose nitrate membrane so as to form an inspection strip T and a quality control strip C; a quantum dot-labeled rubella virus IgG monoclonal antibody is located at the other end of the glass cellulose membrane B and corresponds to the inspection strip T and the quality control strip C, and the quantum dot-labeled rubella virus IgG monoclonal antibody is located at one end of a sample feeding point. The inspection sensitivity of the method is higher than of the currently used method by about 1000 times.
Owner:魏县聚邦新材料科技有限公司
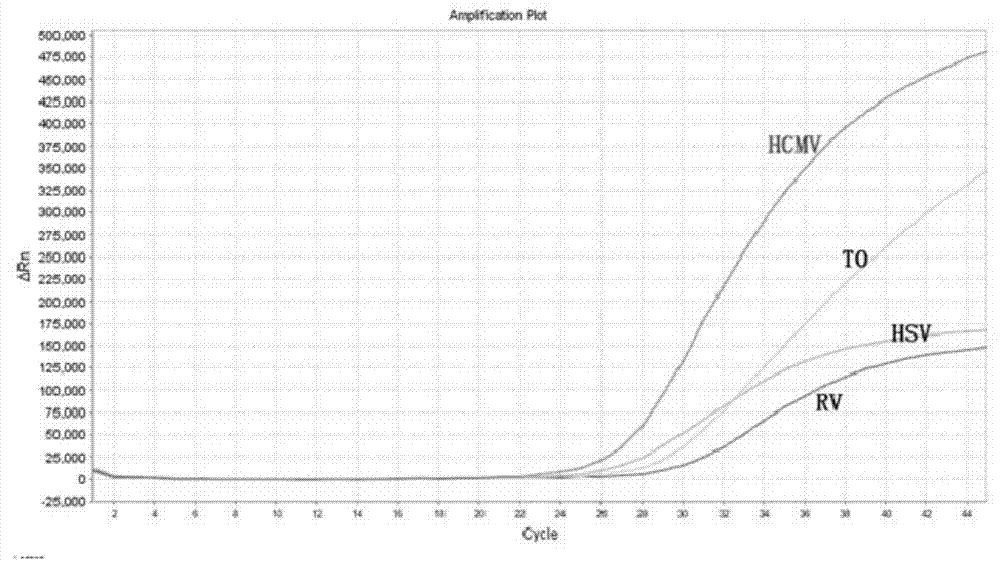
Polymerase chain reaction (PCR) method for identifying four pathogens in prenatal and postnatal care examination through single tube and kit thereof
ActiveCN104120195AAccurate detectionReliable detectionMicrobiological testing/measurementDNA/RNA fragmentationReaction tubeToxoplasma gondii
The invention discloses a real-time fluorescent polymerase chain reaction (PCR) method for simultaneously detecting four targeting nucleic acids in a single PCR reaction tube. The method is used for detecting toxoplasma gondii, rubella virus, cytomegalovirus and herpes simplex virus in samples. The method is fast in operation, is finished through one step and is high in accuracy and sensitivity, and false positive and false negative results are not discovered in practice detection. In addition, the invention also relates to a reagent involved in the PCR method, as well as a detection kit for the method and preparation and application of the detection kit.
Owner:苏州华益美生物科技有限公司
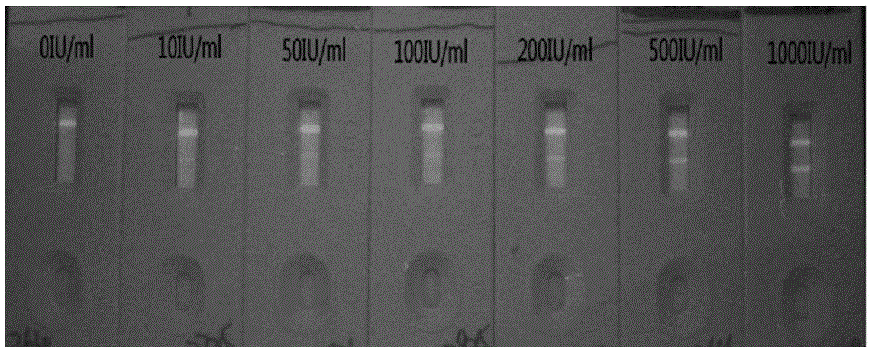
Immunochromatographic kit for quantitative detection of RV (rubella virus) IgG (immunoglobulin G) antibody through quantum dots
InactiveCN105067584AEasy to operateHigh sensitivityFluorescence/phosphorescencePolyesterNitrocellulose
The invention discloses an immunochromatographic kit for quantitative detection of an RV (rubella virus) IgG (immunoglobulin G) antibody through quantum dots. The immunochromatographic kit comprises a detection strip, series of standards and a sample diluent, wherein the detection strip comprises absorbent paper, a nitrocellulose membrane, a conjugate pad and a sample pad which are bonded to a liner plate sequentially; a detection zone of the nitrocellulose membrane is coated with an RV purified antigen, and a quality control zone of the nitrocellulose membrane is coated with a goat-anti-mouse IgG polyclonal antibody; the conjugate pad is formed by spraying a quantum dot labeled mouse-anti-human IgG monoclonal antibody solution onto a polyester glass fiber and drying the polyester glass fiber into a solid state. The immunochromatographic kit has the advantages as follows: a quantum dot quantification technology and immunochromatography are combined, the operation is simple and rapid, and limit of the sample number is avoided, the sensitivity is higher than that of a colloidal gold immunochromatographic method, RV IgG antibodies in human whole blood, serum and blood plasma can be detected quantitatively, and RV-IgG antibody level detection can be performed on progestational women more conveniently.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Human embryonic lung fibroblastic cell SV-7 and application thereof
ActiveCN103387958ANo pollution in the processEasy to trainViruses/bacteriophagesEmbryonic cellsEnterovirusRotavirus RNA
The invention provides a human embryonic lung fibroblastic cell SV-7 with collection number CGMCC No.6956 and an application thereof. The SV-7 cell has obvious characteristics, vigorous growth and long life cycle; the average population doubling level is the 60th generation; the SV-7 cell is pure without pollution, and the culture method is simple; and moreover, the SV-7 cell has adaptability to multiple viruses, can be used for culturing varicella-zoster virus, enterovirus type 71, poliovirus, coxsackie virus A16, rubella virus, hepatitis A virus, rabies virus, rotavirus and measles virus, and can be further used for developing and preparing vaccines against the viruses. The cell culture cost is low, and the SV-7 has good economic values and broad application prospects.
Owner:SINOVAC RES & DEV
Recombinant rubella virus protein and uses thereof
ActiveCN101509002AStrong specificityImproving immunogenicityGenetic material ingredientsMicrobiological testing/measurementRubulavirus InfectionsNucleotide
The invention relates to a rubella virus protein for clinical diagnosis of rubella virus infection and a nucleotide sequence encoding the protein. The protein comprises plasmids of the nucleotide sequence and procaryotic host cells. The invention also comprises a method for preparing the protein and application thereof.
Owner:北京英诺特生物技术股份有限公司
Four-color TORCH (Toxo, others, RV, CMV and HSV) fluorescent detection kit
InactiveCN107058631AHigh detection throughputReduce screening costsMicrobiological testing/measurementMicroorganism based processesPositive controlFluorescence
The invention discloses a four-color TORCH (Toxo, others, RV, CMV and HSV) fluorescent detection kit, comprising a hot start Taq enzyme system, primers, a fluorescent probe, a negative control and a positive control; the fluorescent probe is a TORCH-specific MGB (minor groove binder) probe. The four-color TORCH fluorescent detection kit is suitable for qualitatively detecting toxoplasma, rubella virus, cytomegalovirus, and herpes simplex virus type I and type II in serum and plasma samples. The four-color TORCH fluorescent detection kit detects toxoplasma, rubella virus, cytomegalovirus, and herpes simplex virus type I and type II in a same tube amplification system by means of multiple fluorescence PCR (polymerase chain reaction) technique. The four-color TORCH fluorescent detection kit has high detection flux and has greatly reduced screening cost.
Owner:GUANGZHOU HEAS BIOTECH CO LTD
Fusion protein for detecting rubella virus antibody and preparation method of fusion virus protein
InactiveCN107098980AIncrease productionImprove featuresSsRNA viruses positive-senseAntibody mimetics/scaffoldsRubella virus antibodyVirus Protein
The invention belongs to the technical field of biological detection and particularly provides fusion protein for detecting rubella virus antibody. The fusion protein includes rubella virus E1 protein fragment I, rubella virus E1 protein fragment II and rubella virus E1 protein fragment III. The invention further provides a preparation method of the fusion protein. The fusion protein has high sensitivity and specificity, and detection rate can be greatly improved by using the fusion protein to detect the rubella virus antibody.
Owner:WEIFANG HIGHTOP BIOTECH
IgG antibody detection kit of TORCH five items and preparation of kit
InactiveCN103018443ASample requirement is smallStrong specificityMaterial analysisPathogenic microorganismTorch
The invention relates to a detection reagent of pathogenic microorganism, and particularly relates to an IgG antibody detection kit of TORCH five items and preparation of the kit. The detection kit is used for detecting IgG antibodies of TORCH five items, namely toxoplasma gondii (TOX), rubella virus (RV), cytomegalovirus (CMV) and herpes simplex 1-type and 2-type viruses (HSV-I / II) in a sample.
Owner:BEIJING KINGHAWK PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com