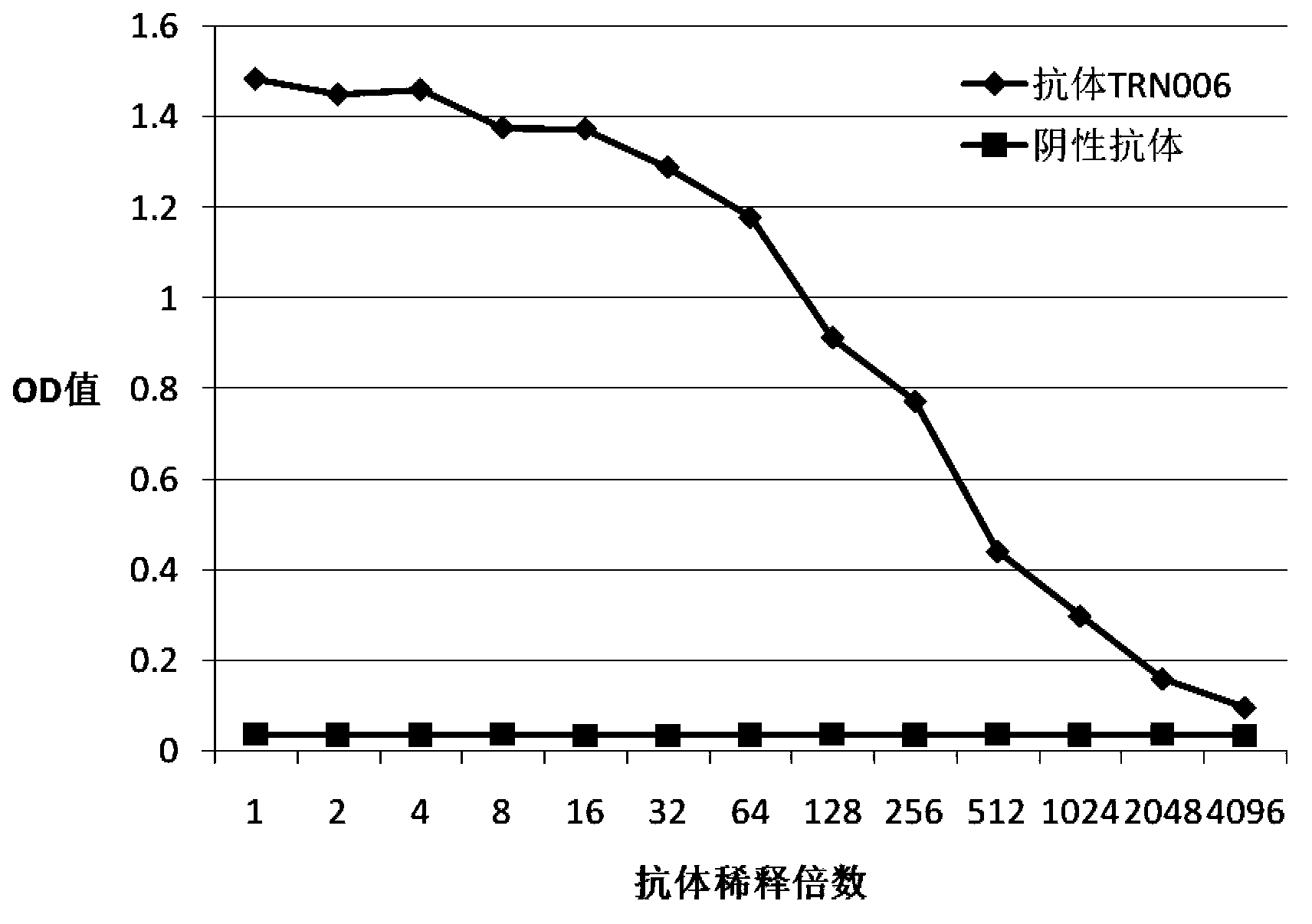
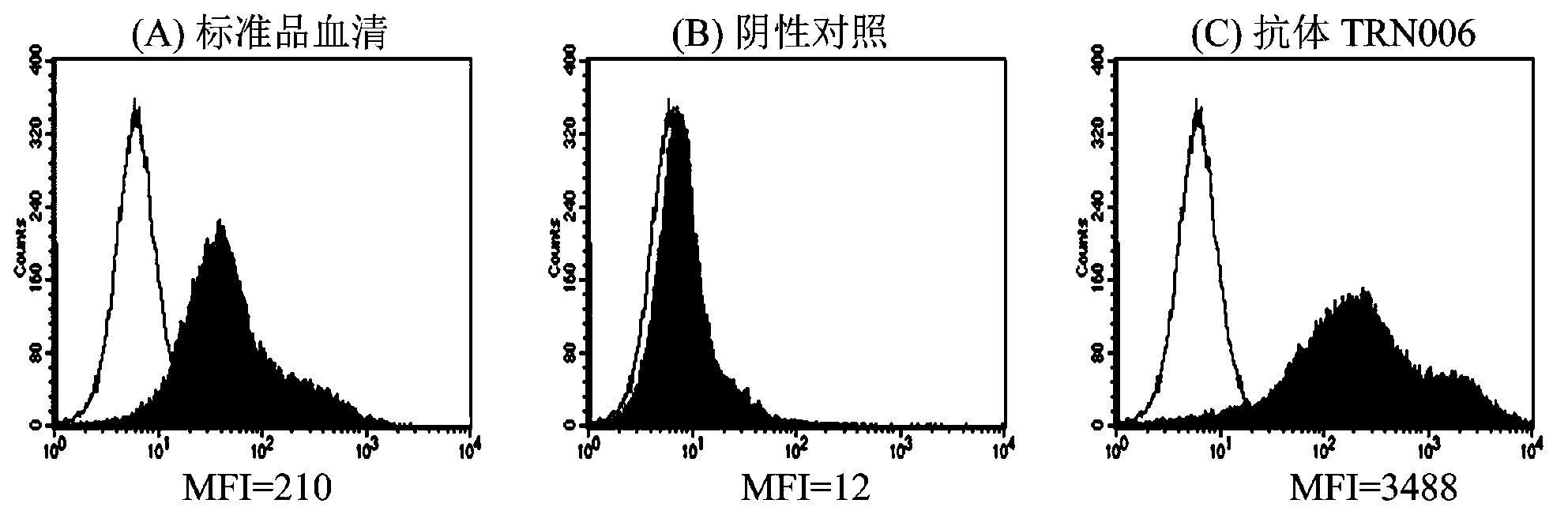
Completely humanized neutralizing antibody for anti-rabies viruses
A rabies virus, fully human technology, applied in the fields of cellular immunology and molecular biology, can solve the problems of long production cycle, limited success rate and low antibody affinity, and achieve good neutralization effect, high affinity and good specificity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] The preparation process of the fully human monoclonal anti-rabies virus neutralizing antibody TRN006 described in the embodiments of the present invention comprises the following steps:
[0019] 1. Lymphocyte Isolation and Single Memory B Cell Sorting
[0020] Collect 100ml of venous blood from two volunteers who have been injected with rabies vaccine (Novartis batch number: 1928) and have produced protective antibodies, put them in anticoagulant tubes containing heparin, and use density centrifugation to separate mononuclear cells (PBMCs) ), after cell counting, use BD FACSria flow cytometer (BD Biosciences, San Jose, CA) to sort CD3-, CD14-, CD16-, CD19+, CD27+ single B cells from PBMC into 96-well PCR containing RT reaction system In the plate, each well contains one memory B cell, and the PCR plate is frozen at -80°C for later use.
[0021] 2. Isolation of Antibody Variable Region Genes from Single B Cells by RT-PCR
[0022] 1) Synthesis of the first strand of c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com