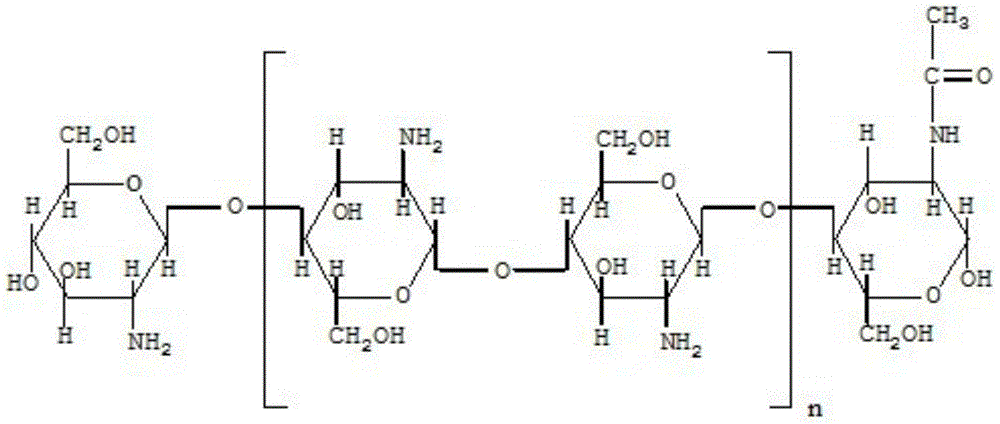
Fosfomycin sodium composition lyophilized powder for injection
A technology of fosfomycin sodium and freeze-dried powder injection, which is applied in the field of pharmaceuticals and pharmaceutical manufacturing, can solve the problems of β-lactamase instability and reduced sensitivity of Klebsiella pneumoniae, and achieve the elimination of activity and distribution in the body Good, the effect of increased stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1, preparation of fosfomycin sodium composition freeze-dried powder for injection, in 1000 pieces.
[0030] prescription:
[0031] Fosfomycin Sodium 80g
[0032] Chitosan Nanoparticles 72g
[0033] Water for injection 2000ml
[0034] 2. Preparation process:
[0035] The chitosan nanoparticle that takes by weighing 72g is slowly added in the water for injection of 2000ml, stirs while adding to dissolve.
[0036] Add 80 g of fosfomycin sodium and stir to dissolve until clear.
[0037] Adjust the pH to 5.1 with buffer salts of sodium dihydrogen phosphate and disodium hydrogen phosphate, add 0.1% activated carbon and stir for 30 minutes, filter out the activated carbon, and filter the liquid through 0.45 μm and 0.22 μm microporous membranes to detect the content of intermediates , according to fosfomycin sodium 80mg per bottle to calculate the filling volume.
[0038] Fill according to the test requirements, put it into a freeze dryer after half-tamping, co...
Embodiment 2
[0039] Embodiment 2, preparation of fosfomycin sodium composition freeze-dried powder for injection, in 1000 pieces.
[0040] 1. Prescription:
[0041] Fosfomycin Sodium 80g
[0042] Chitosan Nanoparticles 81g
[0043] Water for injection 2000ml
[0044] 2. Preparation process:
[0045] The chitosan nanoparticle that takes by weighing 81g is slowly added in the water for injection of 2000ml, stirs while adding to dissolve.
[0046] Add 80 g of fosfomycin sodium and stir to dissolve until clear.
[0047] Adjust the pH to 5.1 with buffer salts of sodium dihydrogen phosphate and disodium hydrogen phosphate, add 0.1% activated carbon and stir for 30 minutes, filter out the activated carbon, and filter the liquid through 0.45 μm and 0.22 μm microporous membranes to detect the content of intermediates , according to fosfomycin sodium 80mg per bottle to calculate the filling volume.
[0048] Fill according to the test requirements, put it into a freeze dryer after half-tamping,...
Embodiment 3
[0049] Embodiment 3, the preparation of fosfomycin sodium composition freeze-dried powder for injection, in 1000 pieces.
[0050] prescription:
[0051] Fosfomycin Sodium 80g
[0052] Chitosan Nanoparticles 64g
[0053] Water for injection 2000ml
[0054] 2. Preparation process:
[0055] The chitosan nanoparticle that takes by weighing 64g is slowly added in the water for injection of 2000ml, stirs while adding to dissolve.
[0056] Add 80 g of fosfomycin sodium and stir to dissolve until clear.
[0057] Adjust the pH to 5.1 with buffer salts of sodium dihydrogen phosphate and disodium hydrogen phosphate, add 0.1% activated carbon and stir for 30 minutes, filter out the activated carbon, and filter the liquid through 0.45 μm and 0.22 μm microporous membranes to detect the content of intermediates , according to fosfomycin sodium 80mg per bottle to calculate the filling volume.
[0058] Fill according to the test requirements, put it into a freeze dryer after half-tamping...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com