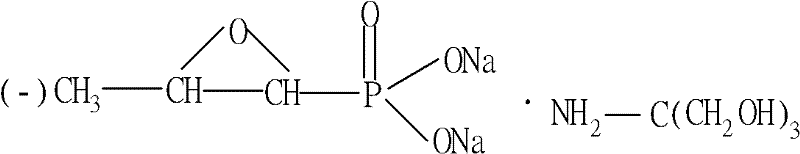
Preparation method of fosfomycin monoamine butantriol
A technology of monotromethamine and bistromethamine, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve incomplete reactions, easy hydrolysis, intermediates Fosfomycin acid instability and other problems, to achieve the effect of simple reaction, high product content, great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 480ml of anhydrous methanol to the reaction bottle, add 80g of fosfomycin bis tromethamine salt under stirring, control the temperature at 20°C, add sulfuric acid and adjust the pH to 6.7, stir for half an hour, filter, add 1000ml of ethyl acetate to the filtrate After crystallization occurs, keep warm (at a temperature of 20° C.) and stir for 2 hours, filter, wash with 10 ml of ethyl acetate, drain and dry (put in an oven for drying at a constant temperature of 40° C.) to obtain 47.5 g of fosfomycin monotromethamine , Yield: 87.0%. The content of fosfomycin monotromethamine detected by liquid phase HPLC was 99.5%.
Embodiment 2
[0021] Add 500ml of anhydrous methanol to the reaction bottle, add 80g of fosfomycin bis tromethamine salt under stirring, control the temperature at 15°C, add trichloroacetic acid and adjust the pH to 6.2, stir for half an hour, filter, and add methyl acetate to the filtrate 1200ml of ester, after crystallization occurs, keep warm (at 20°C) and stir for 2 hours, filter, wash with 10ml of methyl acetate, drain and dry (put in an oven at 40°C for constant temperature drying) to obtain fosfomycin monotromethamine 47.1 g, yield: 86.3%. The detected content of fosfomycin monotromethamine was 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com