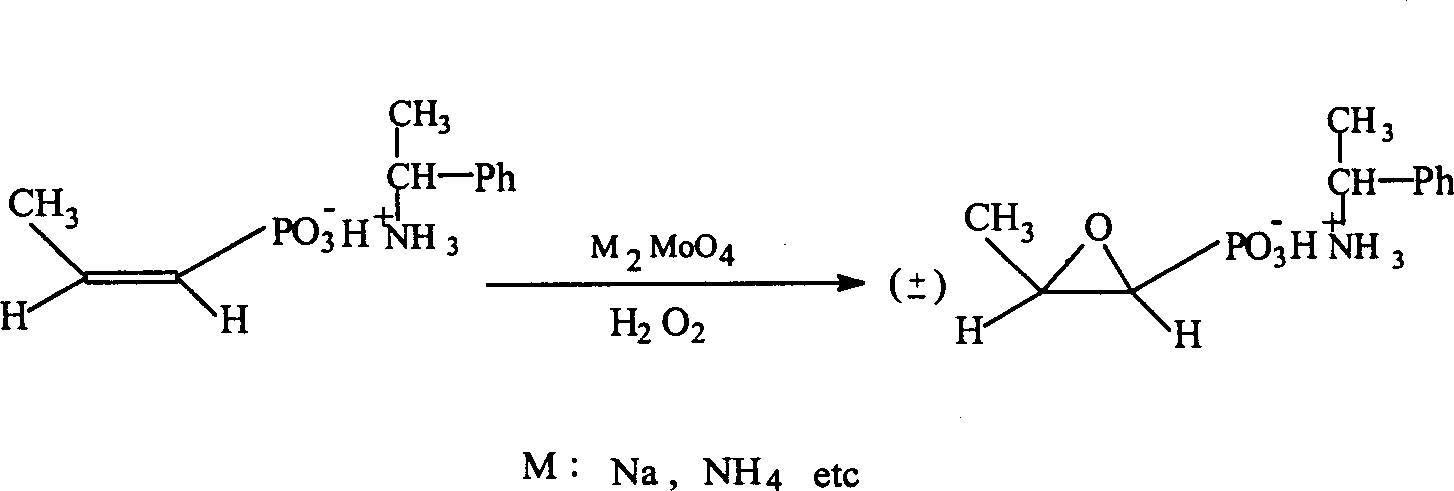
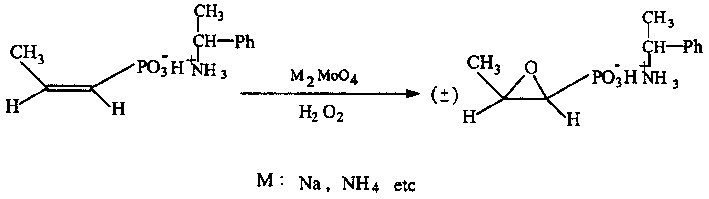Process for synthesizing fosfomycin using cis-propenyl phosphonic acid as raw material
A technology of cis-propenylphosphonic acid and fosfomycin, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problems of high price of catalysts and oxidants, inconvenient use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1. Dissolve 10 g of cis-acrylphosphonic acid (content 83%, industrial product) in 30 ml of ethanol, and slowly add about 9 ml (+)-α-phenylethylamine dropwise at 25 ° C with stirring to make the mixture PH value is 5.5-6.0;
[0021] 2. Dissolve 0.4g of sodium molybdate in 2.0ml of water, and add it to the above solution while stirring;
[0022] 3. After heating up to 40°C, slowly add 3.5ml of 30% hydrogen peroxide (H 2 o 2 ), the dropwise addition was completed in 0.5 hours, and the reaction temperature should not exceed 60°C. The reaction was continued at this temperature for 2 hours.
[0023] 4. Put the above reaction solution in the refrigerator at -5°C overnight, filter it with suction, rinse the filter cake with ethanol, and dry it. 7.3 g of (-)-fosfomycin-(+)-α-phenethylamine salt was obtained, with a yield of 75%.
[0024] Elemental analysis (C 11 h 18 NO 4 P·3 / 2H 2 O) C, H, and the deviation between the measured value and the theoretical value of N are w...
Embodiment 2
[0030] 1. Dissolve 10g of cis-acrylphosphoric acid (content 83%, industrial product) in 30ml of deionized water, and slowly add 9ml of (+)-α-phenylethylamine dropwise under stirring at 25°C, so that the pH of the mixture is 5.5-6.0.
[0031] 2. Add 0.4 g of sodium molybdate into the above solution under stirring, and stir for 10 minutes.
[0032] 3. Raise the temperature to 40-45°C and slowly add 3.5ml of 30% H 2 o 2 , After 0.5 hours, the dropwise addition is completed, and the reaction temperature should not exceed 65°C. Thereafter the reaction was continued at 60°C for 1.5 hours.
[0033] 4. Vacuum remove 70% of the water from the above reaction solution, add 30ml of 95% ethanol, dissolve, stir evenly, and place in the refrigerator at -5°C overnight. Suction filtration, the filter cake was rinsed with ethanol and dried. 7.4 g of (-)-fosfomycin-(+)-α-phenethylamine salt was obtained, with a yield of 76%.
[0034] Elemental analysis (C 11 h 18 NO 4 P·3 / 2H 2 O) C, H,...
Embodiment 3
[0039] The sodium molybdate of 0.4g in embodiment 1 is changed into 0.4g ammonium molybdate, and other processing conditions are all identical with embodiment 1, and the yield and quality of gained product are also identical.
[0040] Molybdenum content of (-)-fosfomycin disodium salt measured by atomic absorption spectrophotometer: 13ppm
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com