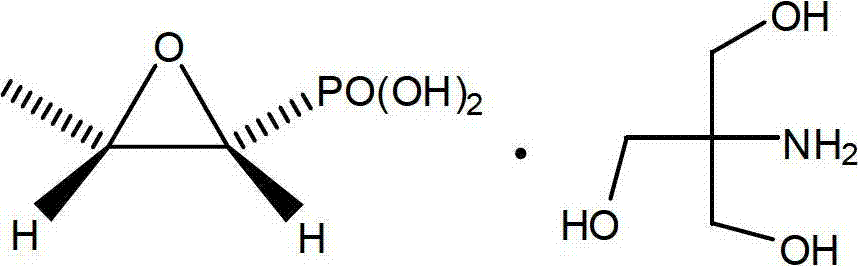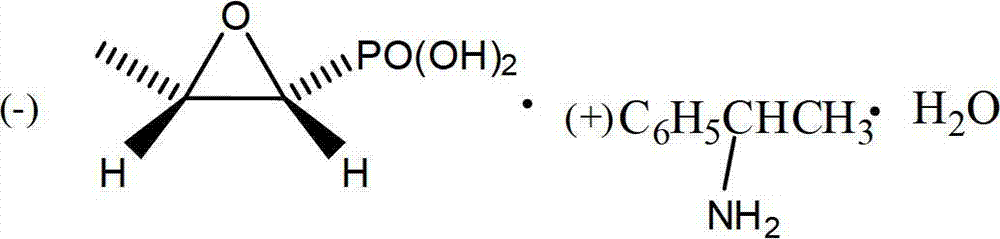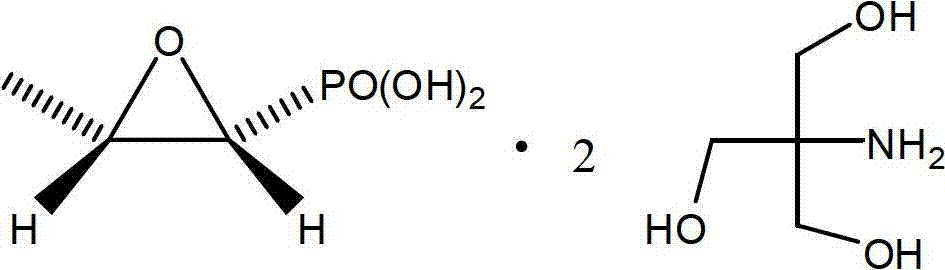Preparation method of fosfomycin amine salt
A technology of fosfomycin and ammonia salt, applied in the field of preparation of fosfomycin ammonia salt, can solve the problems of low yield, cumbersome reaction steps and the like, and achieve the effects of convenient operation, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] In a 500ml three-necked flask, put left phosphorus and right ammonium salt (23.35g, 84.2mmol), tromethamine (10.20g, 84.2mmol) and methanol 170g (215mL), stir at room temperature for 15 minutes, and start to add isomethamine dropwise. Methyl cyanate (4.80g, 84.2mmol) (CAS No. 624-83-9), the dropwise addition was completed in about five minutes, the temperature rose to 33°C, then stirred for 30 minutes, and the methanol was removed by rotary evaporation under reduced pressure, and 200g of ethanol was added. Cool to 0°C, filter, wash with 30g of ethanol, and vacuum dry at 45°C to obtain 18.73g of white solid with a yield of 85.8%. m.p.119~121°C, 1 H NMR (D 2 O): (ppm)=1.33(d,3H,CH 3 ),3.20-2.79(m,2H,CH),3.57(s,6H,CH 2 ).
Embodiment 2
[0040] In a 500ml three-neck flask, put fosfomycin bis tromethamine (32.03g, 84.2mmol) and methanol 170g, stir at room temperature for 15 minutes, start to drop methyl isocyanate (4.80g, 84.2mmol), The dropwise addition was completed in about five minutes, then stirred at room temperature for 30 minutes, and then the methanol was removed by rotary evaporation under reduced pressure, and 200g of ethanol was added, cooled to 0°C, filtered, washed with 30g of ethanol, and vacuum-dried at 45°C to obtain 19.65g of a white solid, with a yield of 90.0 %, m.p.119~121℃.
Embodiment 3
[0042] In a 500ml three-necked flask, put left phosphorus and right ammonium salt (23.35g, 84.2mmol), tromethamine (10.20g, 84.2mmol) and methanol 170g (215mL), stir at room temperature for 15 minutes, and start to drop benzene Ethyl isocyanate (12.39g, 84.2mmol), (CAS No. 1943-82-4) was added dropwise in about 8 minutes, the temperature rose to 33°C, then stirred for 30 minutes, and the methanol was removed by rotary evaporation under reduced pressure, and 200g of ethanol was added, cooled After reaching 0°C, filtered, washed with 30g of ethanol, and vacuum dried at 45°C, 18.32g of white solid was obtained, with a yield of 83.9%. m.p.119~121,
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com