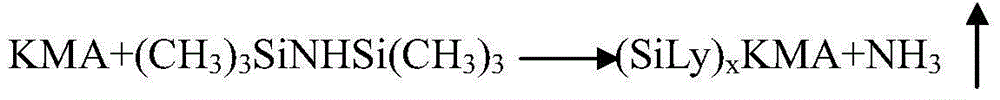A synthetic method of amikacin
A synthetic method, amikacin technology, applied in the field of medicine, can solve the problems of increasing the amount of reaction solvent used, increasing the production cost, and easy volatilization of the solvent, and achieve the effects of simplifying production equipment, improving selectivity, and ensuring synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Put 600mL of acetonitrile into the silanization reaction bottle, put in 0.1 billion kanamycin A (KMA), close the feeding port and start stirring for 10 minutes, add 400mL of hexamethyldisilazane (HMDS), heat up to reflux , Reflux reaction at 75-80°C for 7hr. Use drinking water to cool down outside the reaction bottle to lower the temperature to below 35°C, let it stand still, and naturally stratify. The lower layer was separated and collected to obtain a silyl-protected product.
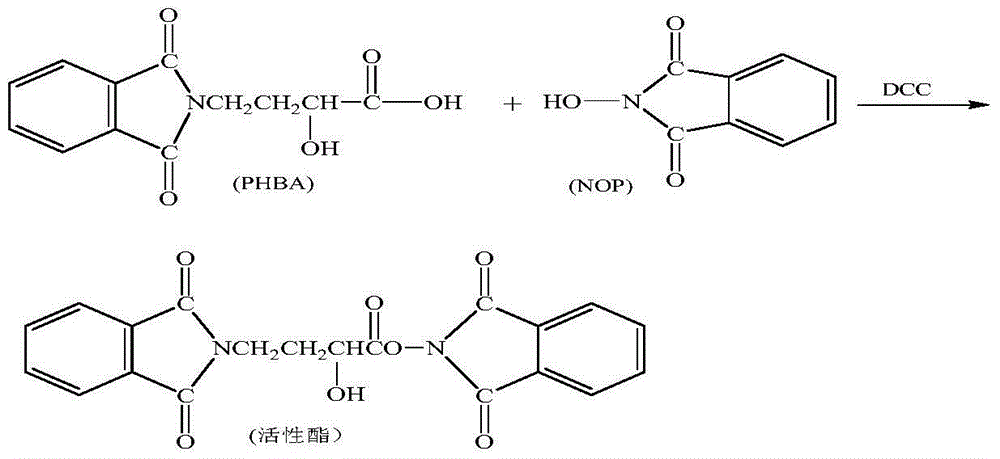
[0047] Add 1000mL of acetone to the silyl-protected product, start stirring, add 60g of γ-N-phthalimide-α-hydroxybutyric acid (PHBA), and then add 2.5g of catalyst 4-N,N-dimethyl Pyridine (DMAP), lower the temperature to -15~-10°C.
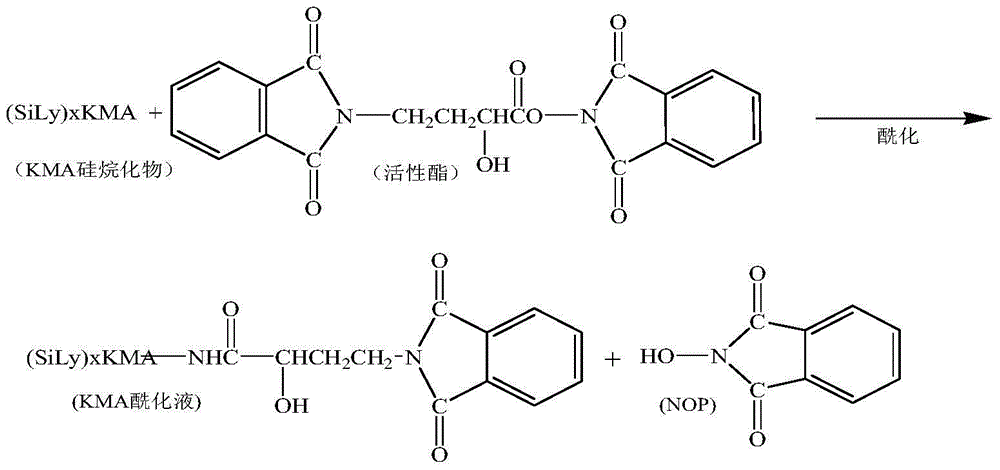
[0048]Dissolve 60g of N,N-dicyclohexylcarbodiimide in 300mL of acetone, and add it to the above reactant, control the flow rate at 5mL / min, and control the temperature of the reactant at -15~-10°C; continue the reaction for 1 hour after the addition is complete ...
Embodiment 2
[0053] Put 600mL of acetonitrile into the silanization reaction bottle, put in 0.1 billion kanamycin A (KMA), close the feeding port and start stirring for 10 minutes, then add 500mL of hexamethyldisilazane (HMDS), heat up to reflux , Reflux at 75-80°C for 8 hours. After the reaction is completed, the temperature is cooled to 40°C with drinking water and left to stand, and the layers are separated naturally. The lower layer was separated and collected to obtain a silyl-protected product.
[0054] Add 1000mL of acetone to the silyl-protected product, start stirring, add 70g of γ-N-phthalimide-α-hydroxybutyric acid (PHBA), and then add 3.0g of catalyst 1-hydroxybenzotriazole ( HOBT), after the material is dissolved, cool down to -15~-10°C.
[0055] Dissolve 70g of N,N-dicyclohexylcarbodiimide in 300mL of acetone, and add it to the above reactant, control the flow rate at 6mL / min, and control the temperature of the reactant at -15~-10°C; continue the reaction for 1.5 hours afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com