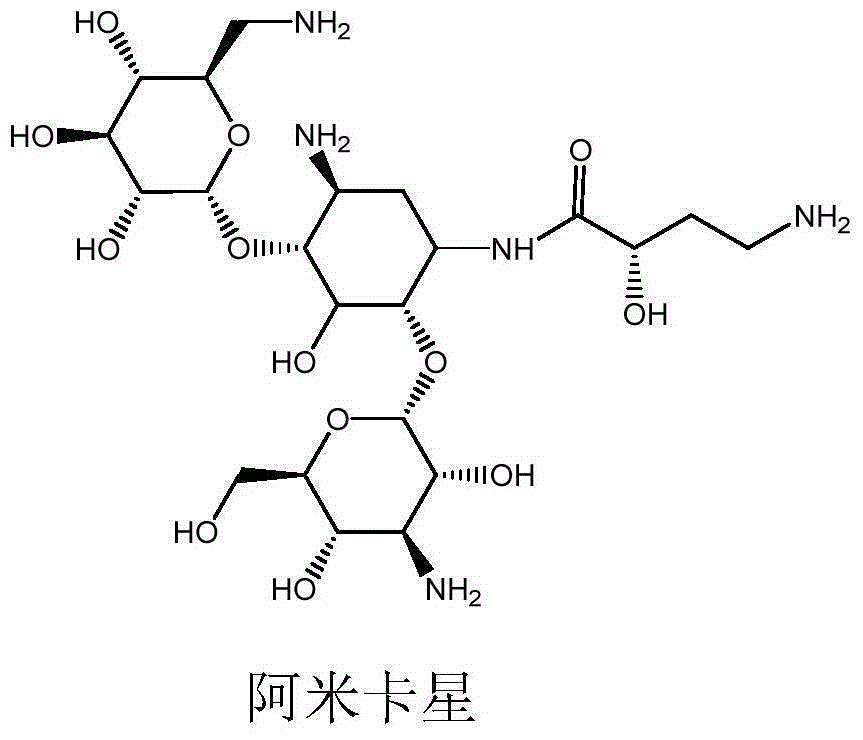
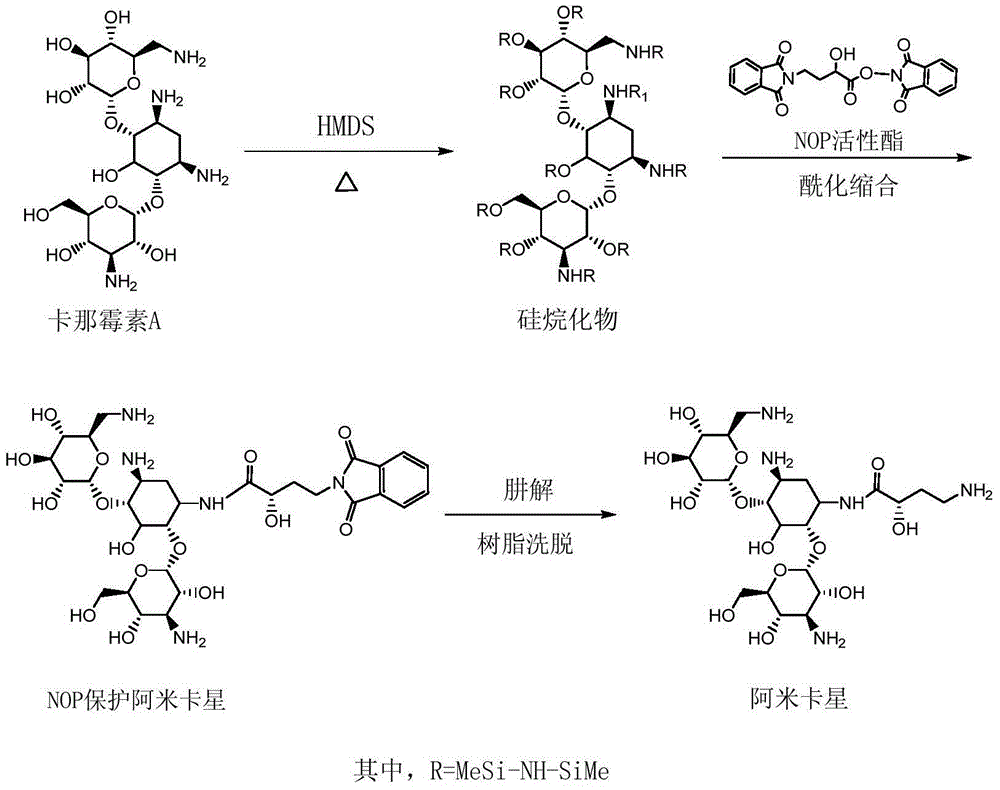
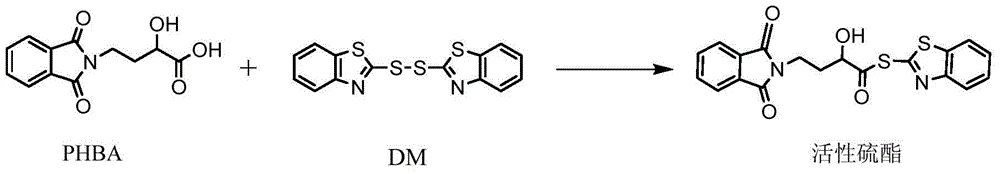
Preparation method of amikacin and intermediate activated thioester thereof
A technology of active thioester and amikacin, applied in the field of medicine, can solve the problems of poor acylation reaction selectivity, many side reactions and high cost, and achieve the effects of high selectivity, easy separation and purification, and reduction of synthesis cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: the preparation of active thioester
[0055] In a 1000ml dry flask, feed in sequence: 200ml of dichloromethane, 50g of PHBA and 100g of DM, and stir thoroughly for 30 minutes; then slowly add dropwise a mixture of 30ml of pyridine, 50ml of triethylamine and 60ml of triethyl phosphite at 5-10°C. Mixed solution, control the rate of addition to drop after 2 hours. Continue to keep warm at 5-10°C for 3-5 hours, then filter the reaction solution, wash the filter cake with dichloromethane; distill off the solvent from the filtrate under reduced pressure, and dry it in vacuum at 50°C to obtain a yellow solid; place the solid in 80ml of methanol Slurry in medium for 1 hour, filter, and vacuum dry at 50° C. to obtain 78 g of a light yellow active thioester solid with a content of >98% and a molar yield of 98%. The mp of the product: 168-172°C. MS (m / z): 398.4; 1 HNMR (DMSO, 300MHz): 2.18(m,2H), 3.381(t,2H), 4.44(t,1H), 5.56(s,1H), 7.58~7.64(d,2H), 7.91~7.91(s, ...
Embodiment 2
[0056] Embodiment 2: the preparation of active thioester
[0057] Add 35g DM, 25g PHBA and 350ml 1,2-dichloroethane into a dry 500ml flask, stir at room temperature for 30 minutes, then cool to 0°C, add 30ml triethylamine dropwise, after the addition is complete, within 2 hours Add 31.5g triphenylphosphine in batches, continue to react for 4 hours after adding, filter, wash the filter cake with 1,2-dichloroethane, distill the solvent from the filtrate under reduced pressure to obtain a yellow solid; add the yellow solid to In 50ml of methanol, stirred at 0°C for 1 hour, filtered, washed with cold methanol, and vacuum-dried at 50°C to obtain 36.5g of light yellow active thioester solid, content>99%, yield 92%.
Embodiment 3
[0058] Embodiment 3: the preparation of active thioester
[0059] Add a mixture of 7.5g PHBA, 12.5g DM, 40g toluene and 60g acetonitrile into a dry flask, stir at room temperature for 30 minutes, then cool down to 10-15°C, add 5ml triethylamine and 7g phosphorous acid within 2 hours The mixed solution of triethyl ester was incubated and reacted for 4 hours; the reaction was completed, the feed liquid was filtered, the filter cake was washed with toluene until colorless, the solvent was distilled under reduced pressure, and then the gained solid was placed in 30ml methanol for beating for 1 hour, filtered to obtain Yellow active thioester solid 11.8g, content>99%, yield 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com