Preparation method of amikacin
A technology of amikacin and kanamycin, which is applied in the field of medicine, can solve the problems of increasing solvent consumption costs, affecting personnel health, and using large solvents, so as to improve operability and safety, realize environmental friendliness, The effect of reducing solvent consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
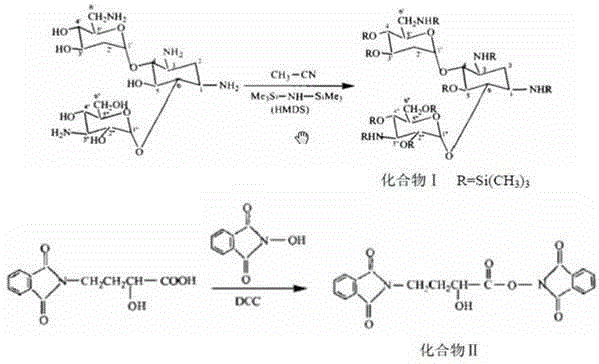
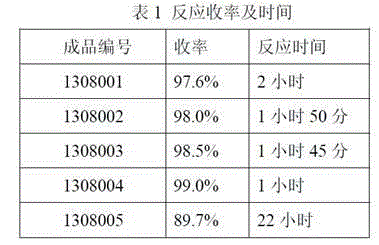
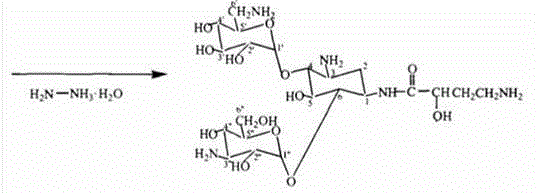
[0029] Take 20g (41.3mmol) kanamycin A, add 66.6g (412.6mmol) hexamethyldisilazane, blow in nitrogen, add 0.2g micronized ammonium sulfate (d0.1=0.5um, d0.9≤ 5um), the temperature was controlled at 50°C, and no ammonia gas was released for 2 hours, evaporated to dryness with a high vacuum pump, and weighed 51.7g (because the product contained 0.2g ammonium sulfate, the calculated yield needs to be deducted, and the weight after deducting is 51.5g), Calculated according to full protection yield 97.5%. (No.: 1308001).
Embodiment 2
[0031] Take 20g (41.3mmol) kanamycin A, add 99.9g (619.0mmol) hexamethyldisilazane, blow in nitrogen, add 0.6g micronized ammonium sulfate (d0.1=0.3um, d0.9≤ 3um), the temperature was controlled at 70°C for reaction, no ammonia gas was released in 1 hour and 50 minutes, evaporated to dryness with a high vacuum pump, and weighed 52.3g (because the product contained 0.6g ammonium sulfate, the calculated yield needs to be deducted, and the weight after deducting is 51.7g ), the yield is 98.0% based on full protection. (No.: 1308002).
Embodiment 3
[0033] Take 20g (41.3mmol) kanamycin A, add 133.2g (825.3mmol) hexamethyldisilazane, blow in nitrogen, add 1.0g micronized ammonium sulfate (d0.1=0.1um, d0.9≤ 1um), the temperature was controlled at 80°C for 1 hour and 45 minutes, no ammonia gas was released, evaporated to dryness with a high vacuum pump, and weighed 53.0g (because the product contained 1.0g ammonium sulfate, the calculated yield needs to be deducted, and the weight after deducting is 52.0g) , The yield is 98.5% based on full protection. (No.: 1308003).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com