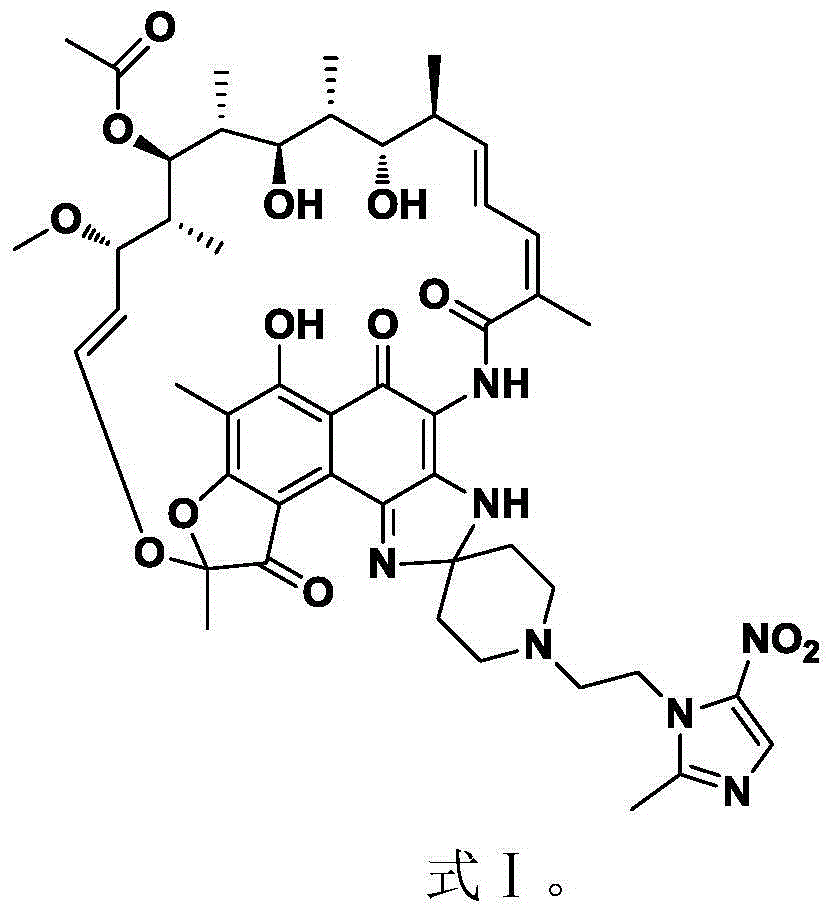
Novel application of rifamycin-nitroimidazole coupling molecule
A technology of nitroimidazole and rifamycin, which is applied in the field of medicinal chemistry, can solve problems such as weak antibacterial activity, and achieve the effect of strong antibacterial effect and strong antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Antibacterial activity of a rifamycin-nitroimidazole conjugate molecule against Helicobacter pylori
[0019] 1.1 Materials and methods
[0020] 1.1.1 Selection of strains
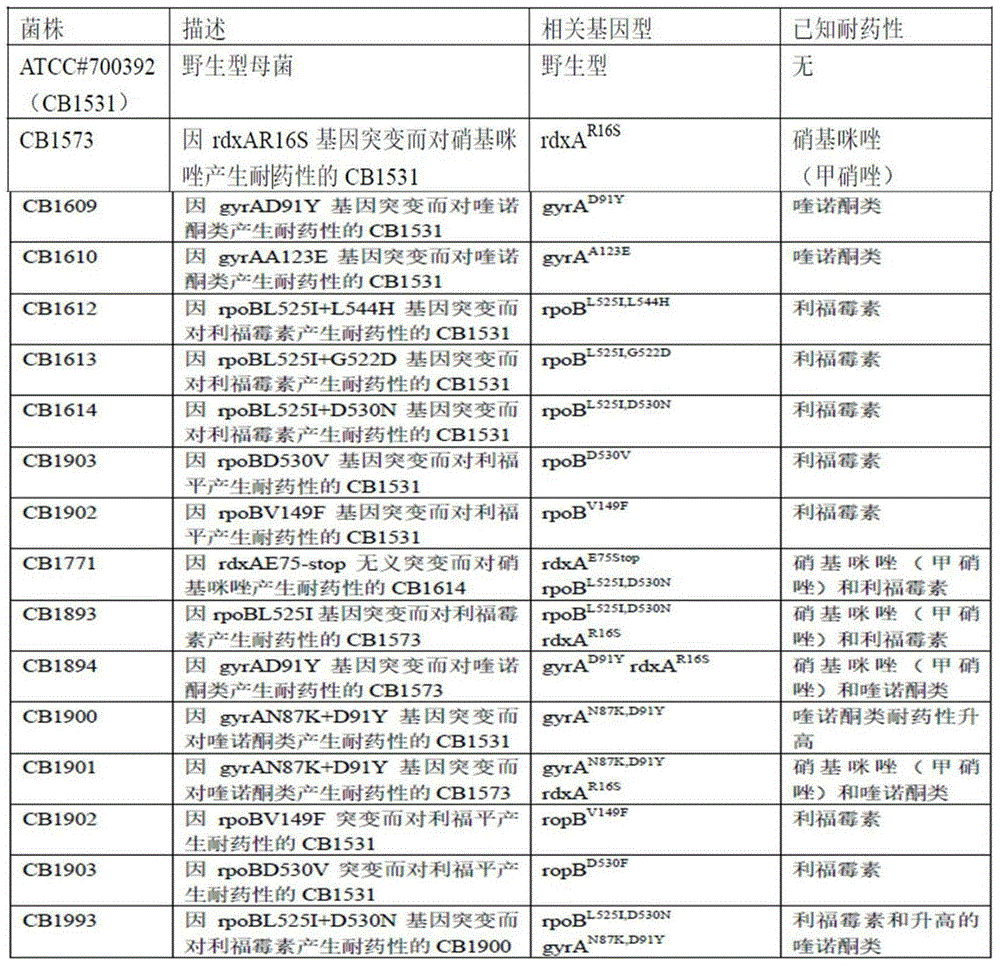
[0021] ATCC#700392 (CB1531) is a wild-type strain, purchased from American Type Culture Collection (ATCC, Manassas, Virginia). CB1573, CB1609, CB1610, CB1612, CB1613, CB1614, CB1771, CB1893, CB1894, CB1900, CB1901, CB1902, CB1903, CB1993 are isogenic mutant strains obtained from CB1531 and carry specific drug resistance genes. Medical supplies. See Table 1 for strain description.
[0022] Table 1 The following strains derived from glycerol stock solution were inoculated on TSAII medium without drugs
[0023]
[0024] 1.1.2 Medium configuration
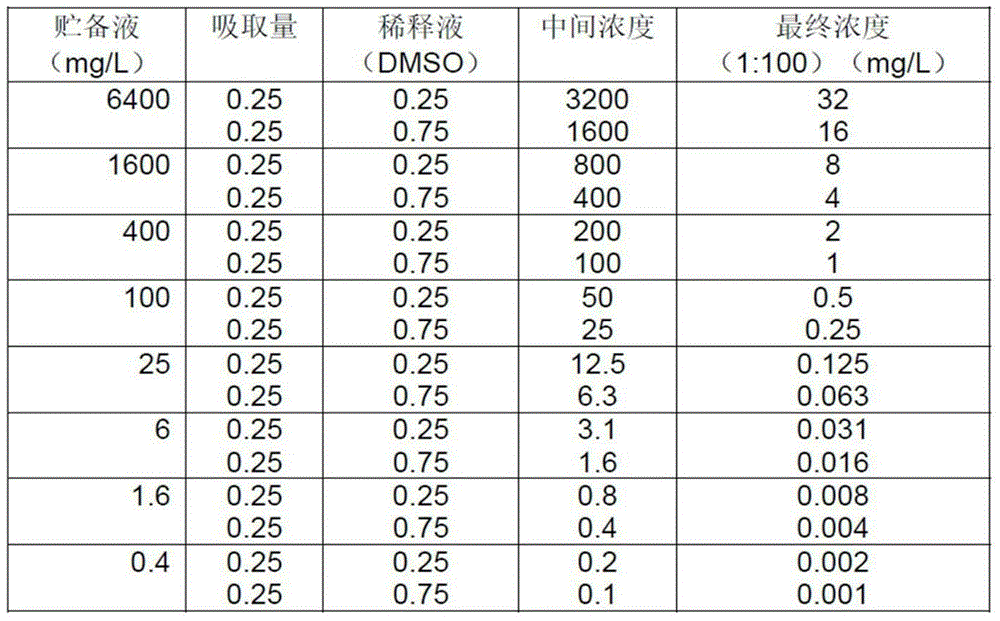
[0025] It was prepared in accordance with the Clinical Laboratory Standards Institute guideline (CLSI) M7-A7 (test method for the determination of the susceptibility of aerobic bacteria to antibiotics by the dilution method, approved standard). The test su...
Embodiment 2
[0047] Example 2 Antibacterial activity of a rifamycin-nitroimidazole conjugate molecule against the Gram-positive anaerobic toxigenic pathogen Clostridium difficile
[0048] 2.1 Materials and methods
[0049] 2.1.1 Selection of strains
[0050] ATCC#BAA-1382 (CB1921) is a toxin-producing Clostridium difficile, purchased from the American Type Culture Collection (ATCC, Manassas, Virginia). CB1934, CB1939, CB1940, CB1941, CB1942 are isogenic strains with specific resistance mutations obtained from CB1921. The test strains were all provided by Dannuo Medicine. See Table 4 for strain description. Table 4 The following strains derived from glycerol stock solution were inoculated on drug-free supplemental Brookfield agar medium
[0051]
[0052] * Note: CB1939 isogenic resistant strains have not been confirmed by sequencing, but CB1942 is a direct descendant of CB1939, indicating that there is at least one mutation in gyrA (D71Y) or (T82A).
[0053] 2.1.2 Medium configuration
[0054] It...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com