One-pot processing method for synthesizing rifampicin
A technology of rifampicin and rifamycin, which is applied in the field of preparation of semi-synthetic rifamycin antibacterial drugs, can solve the problems of affecting the quality of intermediates and finished products, affecting the quality of intermediates, and increasing the number of impurities, so as to save The effect of improving the quality of chemical raw materials and finished products and simplifying the process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
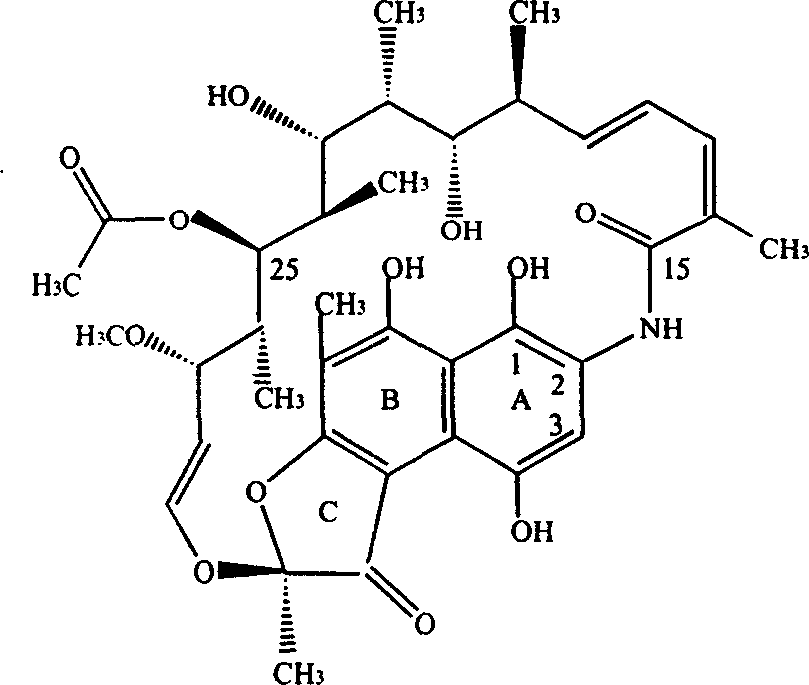
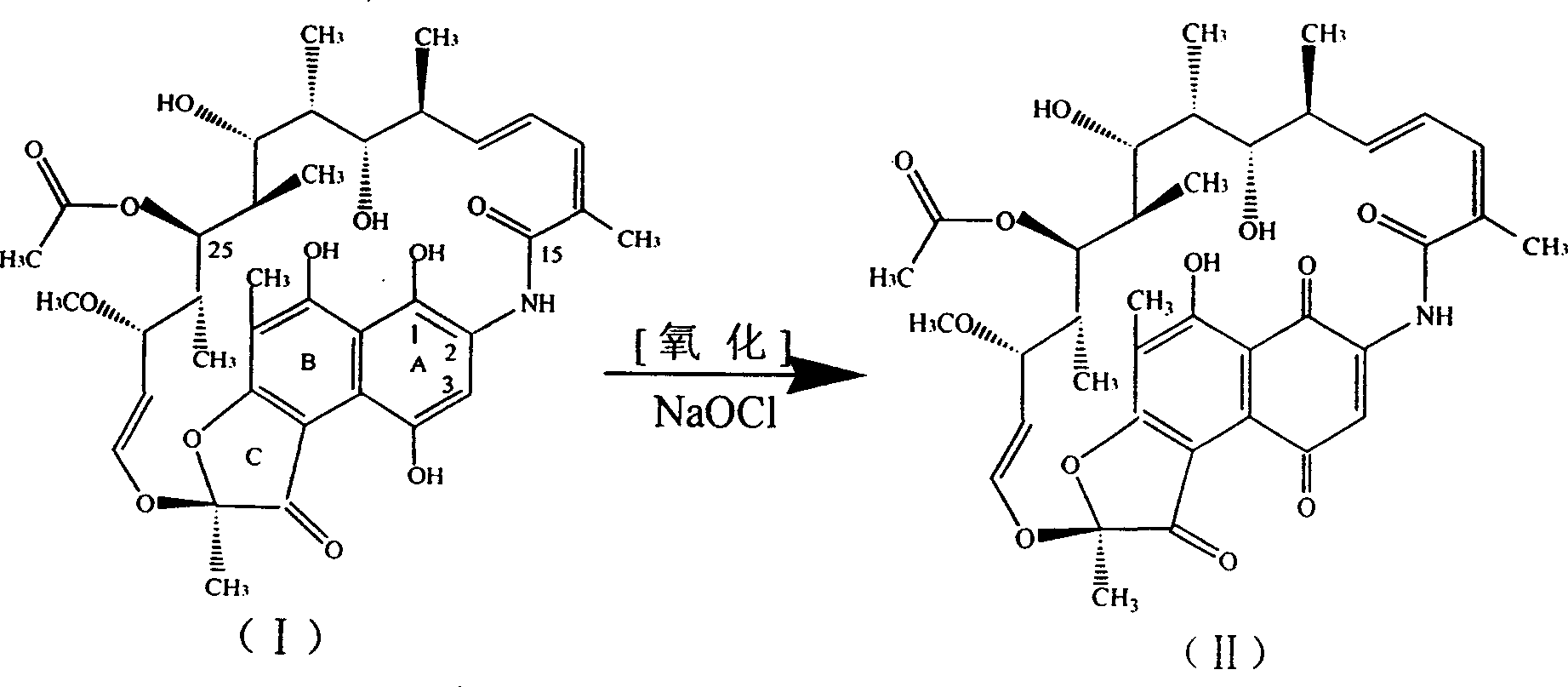
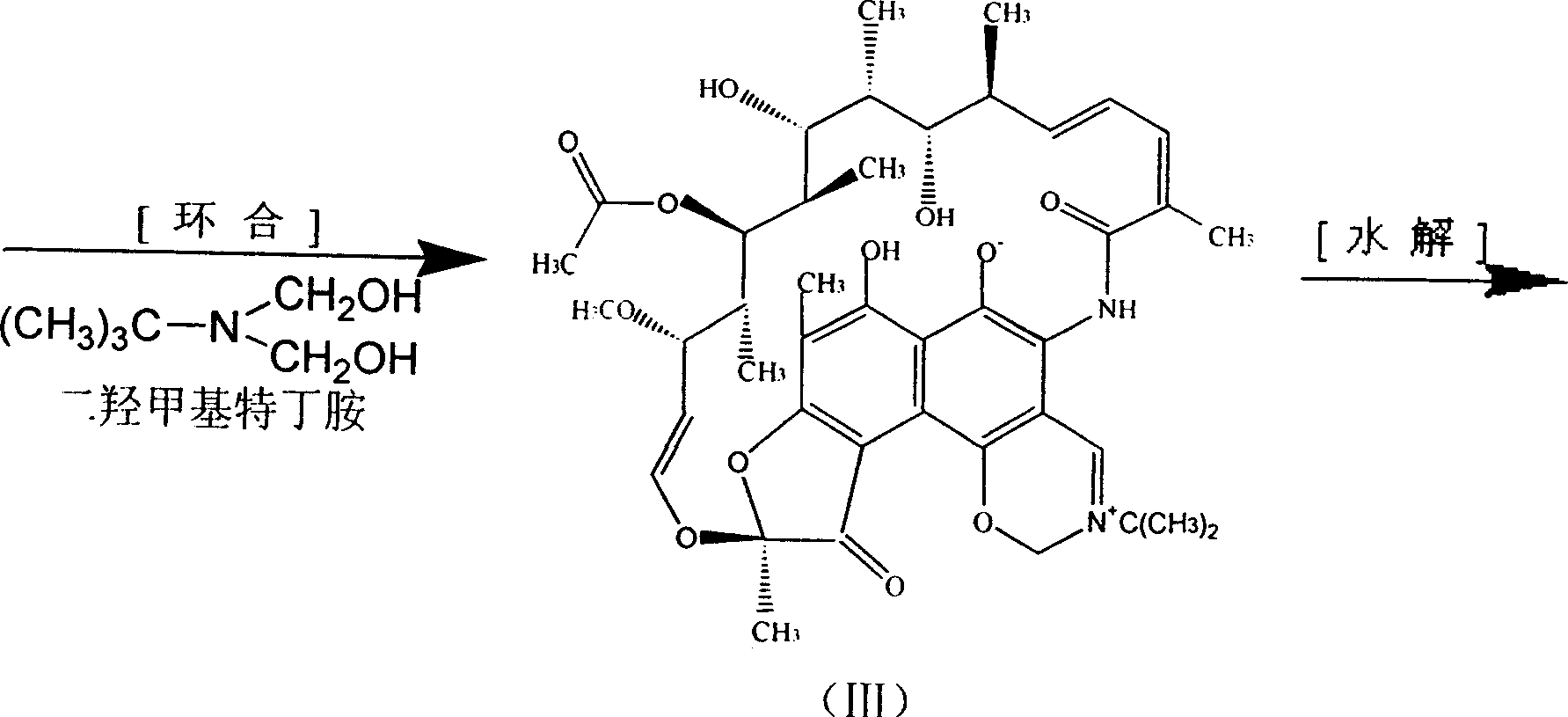
[0035] Get 2520ml of ethyl acetate solution containing rifamycin SV 17.2 million (gained after separation and purification by macroporous resin, 6830u / ml), add dropwise 182 grams of 7.84% sodium hypochlorite aqueous solution under stirring to carry out oxidation reaction, and the reaction solution is passed through thin Layer chromatography, the rifamycin SV point disappears, that is, the ethyl acetate oxidation solution of rifamycin S is generated, first washed with saturated saline, then deionized water, separated to remove the water phase, and the organic phase TLC showed One spot, recovered ethyl acetate, concentrated to dryness under reduced pressure, added 40ml of dimethylformamide to dissolve, added dropwise 8.7g of dimethylol terbutylamine under stirring, and reacted at 50°C for two hours to obtain N-tertbutyl- 1,3-oxazine[5,6-C]rifamycin, one spot on TLC. Recover dimethylformamide under reduced pressure to dryness. After the recovery is complete, add 60ml of n-butanol...
Embodiment 2
[0037] Get 12000ml (3584u / ml) of fermentation filtrate containing rifamycin SV 43 million, add 860ml butyl acetate, dropwise add 460 grams of sodium hypochlorite with a concentration of 7.8%, carry out oxidation reaction to generate rifamycin S, add dropwise under stirring Adjust the pH to 6.0 with 10% dilute hydrochloric acid, let it stand still, and measure that the aqueous phase contains rifamycin S below 30u / ml, separate the aqueous phase, and use 1% NaHCO for the organic phase 3 Wash with aqueous solution, and then wash with deionized water for several times, then recover butyl acetate, concentrate to dryness under reduced pressure, add 60ml of dimethylformamide to dissolve, add 17.7g of dimethylol terbutylamine dropwise under stirring, and react at 50°C for two N-tertidine-1,3-oxazine [5,6-C] rifamycin was obtained in 1 hour, dimethylformamide was recovered under reduced pressure to dryness, then 90 ml of n-butanol was added to dissolve, and 9.0 g of Morpholine, 12.24 gr...
Embodiment 3
[0039] Get 1066ml of butyl acetate solution containing rifamycin SV15.22 million (gained after separation and purification by macroporous resin, 14280u / ml), add dropwise 88 grams of 11.46% sodium hypochlorite aqueous solution under stirring to carry out oxidation reaction, and the reaction solution is passed through Thin layer chromatography shows a spot of rifamycin S, that is, the butyl acetate oxidation solution of rifamycin S is obtained, first washed with salt water, then washed with deionized water, separated to remove the aqueous phase, and organic phase thin layer chromatography A spot appeared, recovered butyl acetate, concentrated to dryness under reduced pressure, then added 35ml of dimethylformamide to dissolve, added dropwise 7.7g of dimethylol terbutylamine under stirring, and reacted at 50°C for two hours to obtain N-tert Buta-1,3-oxazine[5,6-C]rifamycin, one spot on TLC. Recover dimethylformamide under reduced pressure to dryness. After the recovery is complete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com