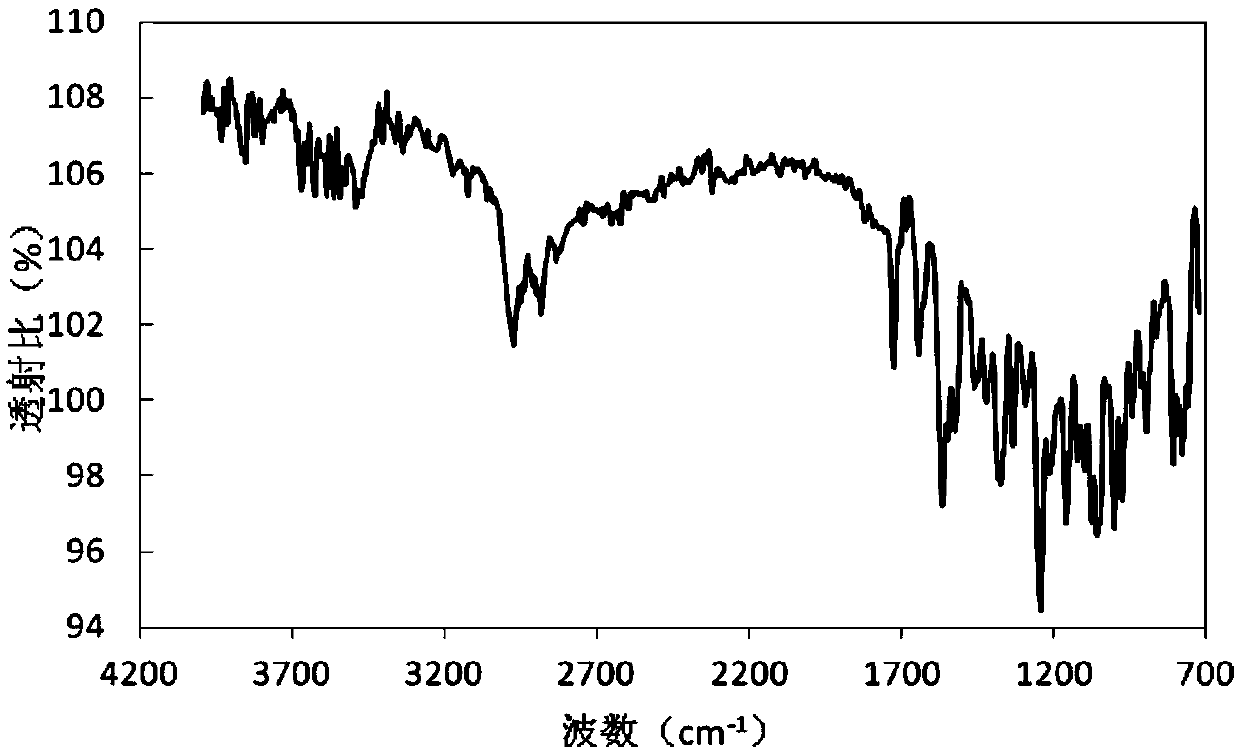
Method for preparing rifampicin I crystal form
A technology of rifampicin and crystal form, which is applied in the field of chemical engineering crystallization, can solve the problems of poor crystal form and fluidity, product stickiness and easy coalescence, high adhesion, etc., to achieve excellent particle size and bulk density, good product fluidity, The effect of short operating time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] The present invention is carried out by the following examples, but is not limited thereto.
[0033] Implementation column 1:
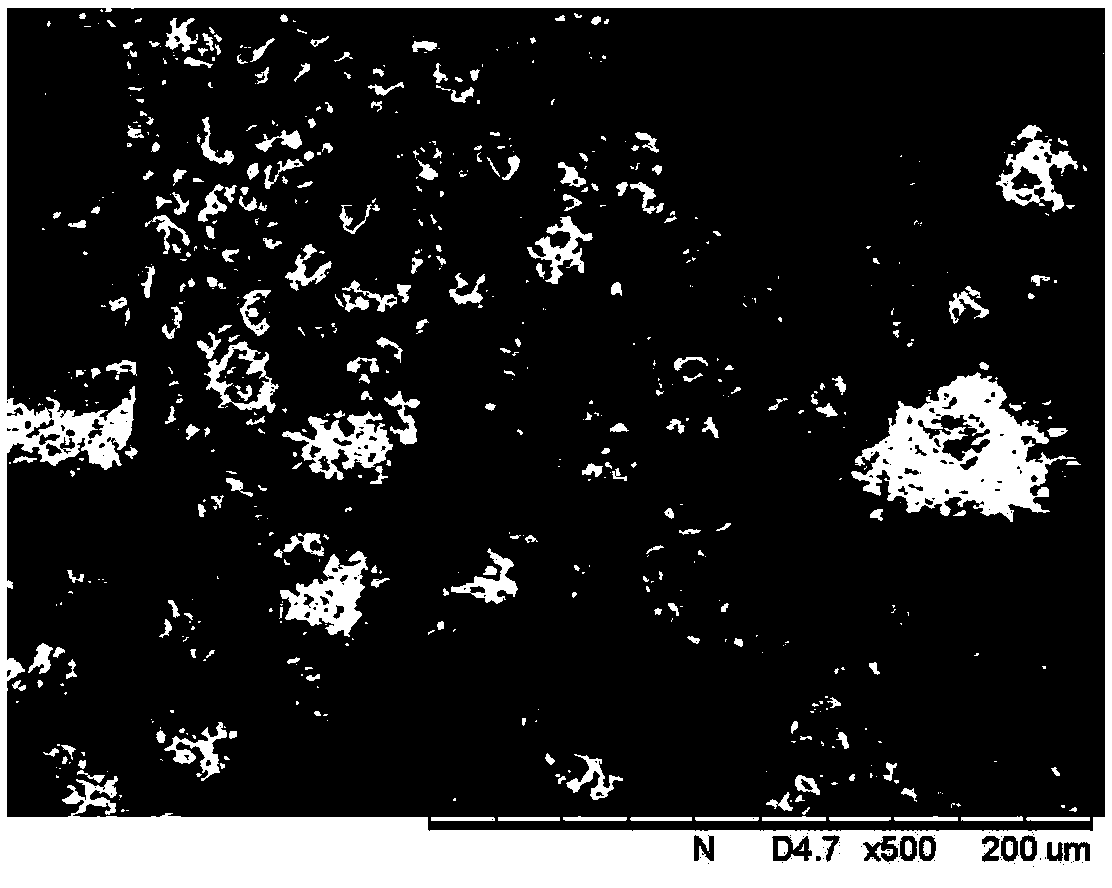
[0034] Dissolve 160 g of crystalline form I rifampicin with a purity of 90% in 1000 mL of butanol under stirring, add 27 mL of water, and raise the temperature to 75° C. to completely dissolve the rifampicin solid; then start vacuum evaporation with a vacuum degree of 0.04 MPa, The evaporation temperature of the solution is 72°C, and the evaporation rate is 170mL / hr. After the crystals are produced, stop the evaporation and grow the crystals for 20 minutes. After the volume of the distillate reaches 500mL, stop the evaporation; Crystal for 20 minutes. The crystalline slurry was filtered, washed, and dried at 35°C for 3 hours to obtain 128.2 g of the crystal form I product of rifampicin, with a yield of 80%.
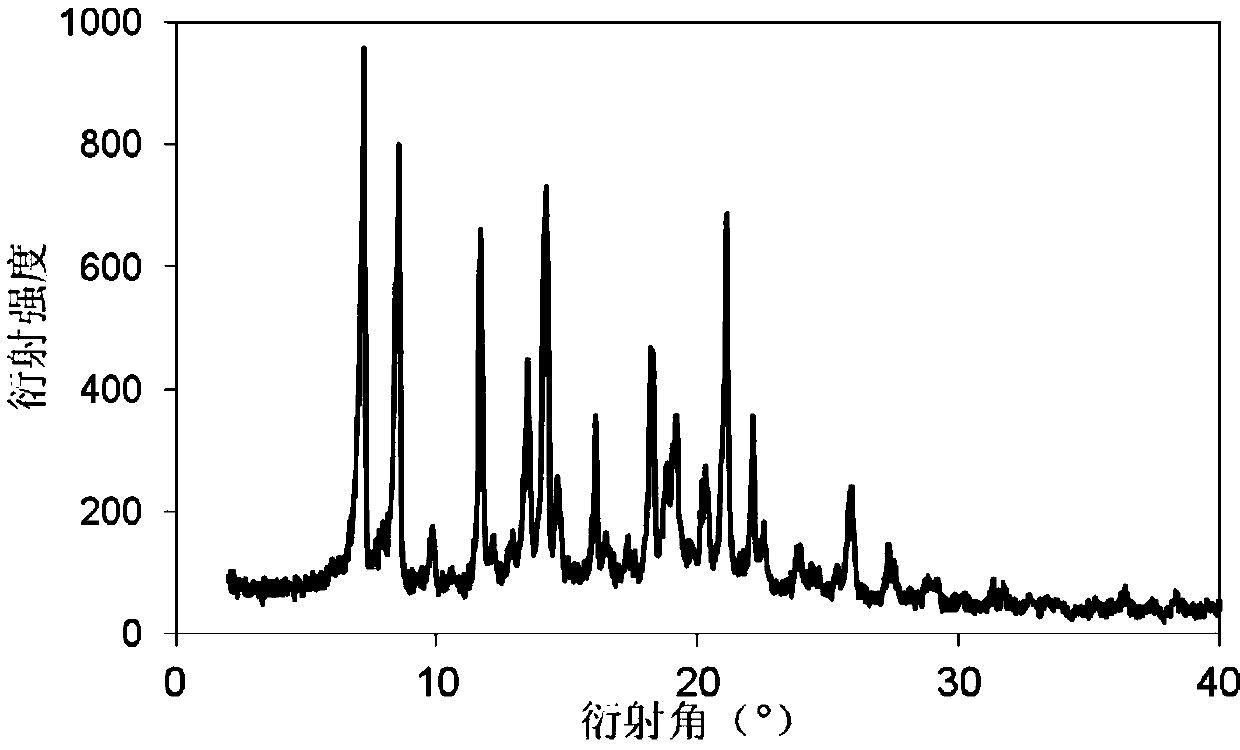
[0035] The powder X-ray diffraction pattern of the product is as figure 2 As shown, there are characteristic peaks at diffraction ang...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com