Patents
Literature
64results about How to "Reduce and prevent occurrence" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
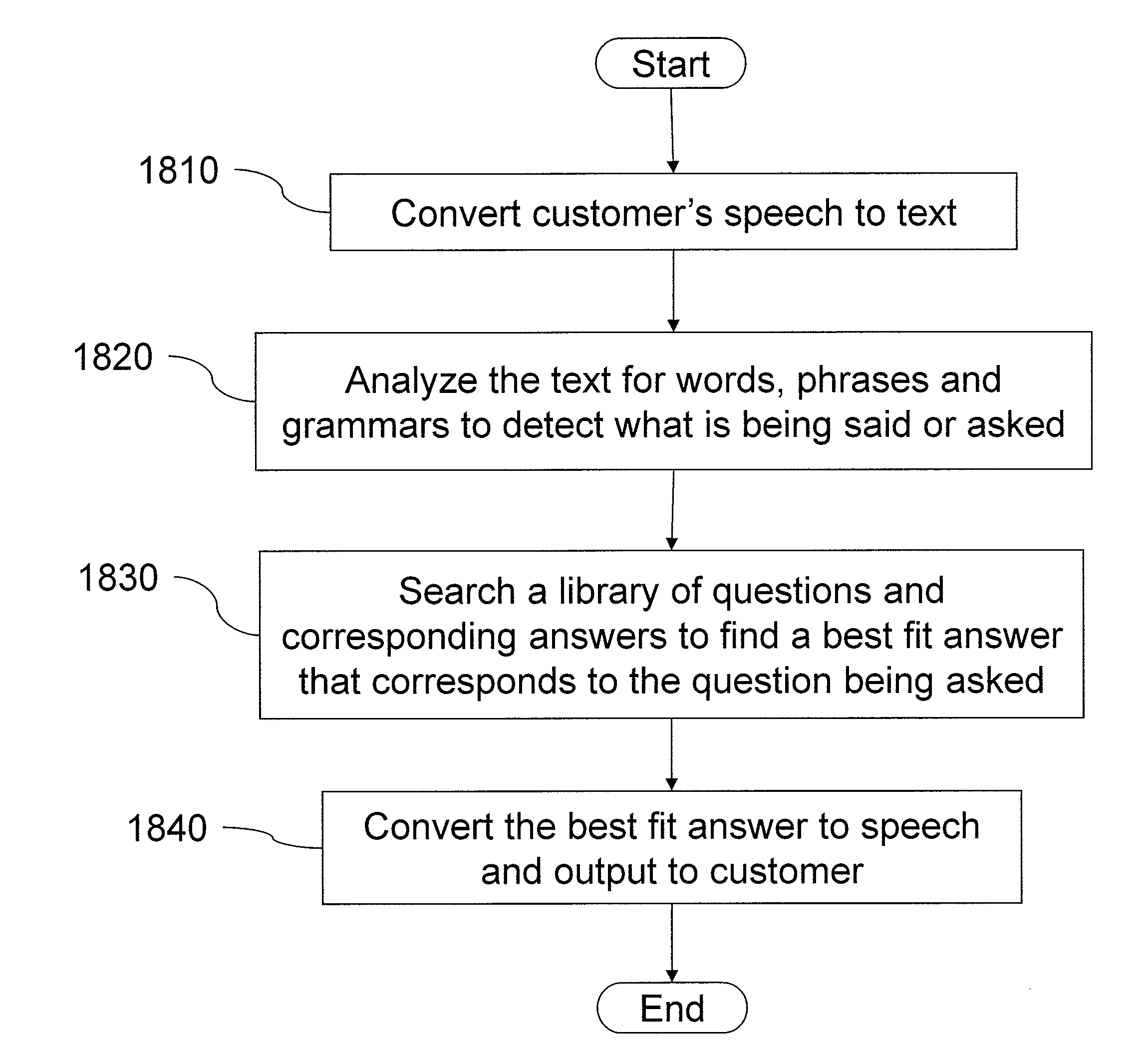
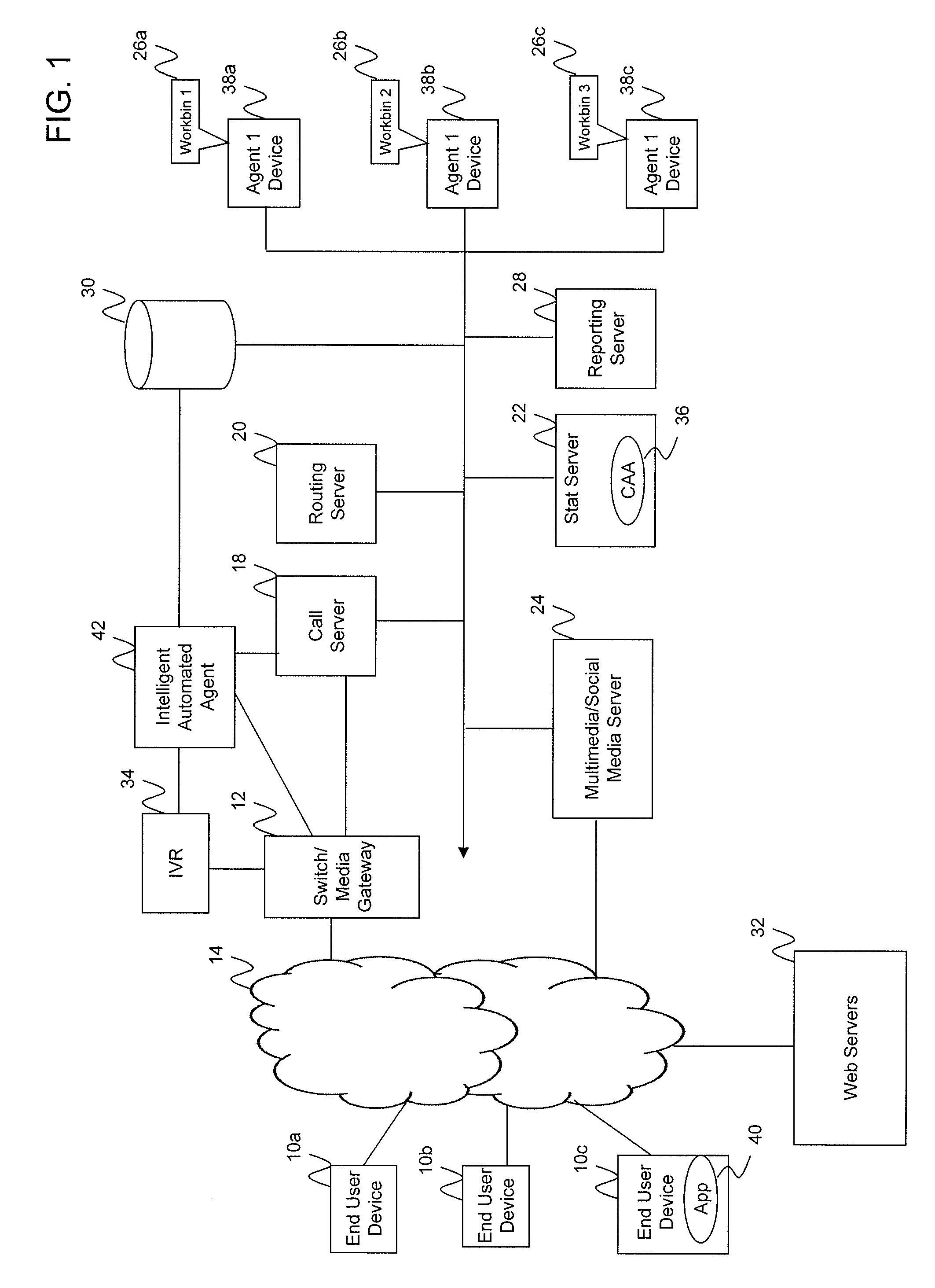
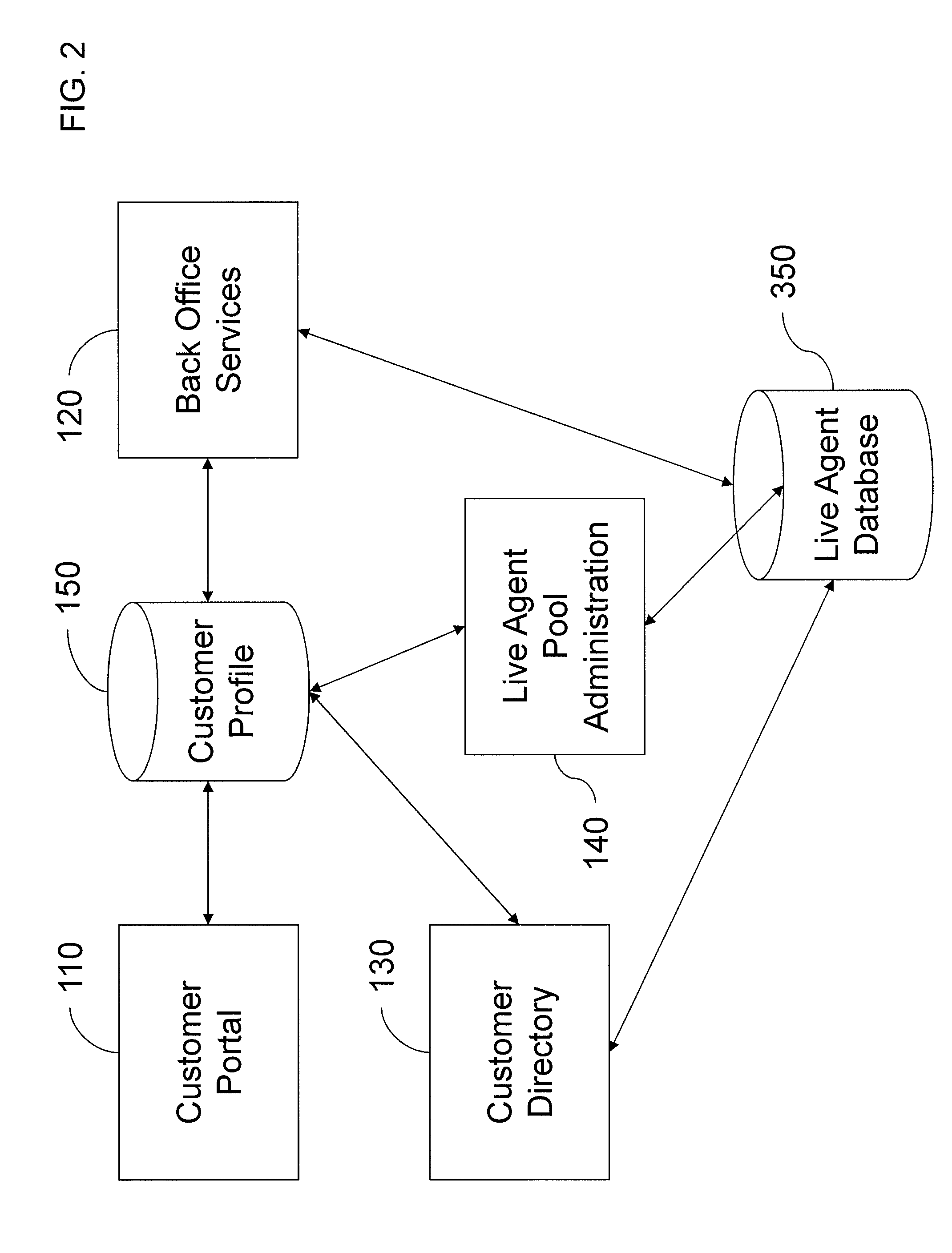
Intelligent automated agent for a contact center
InactiveUS20140314225A1Improve customer serviceGood and more personalized serviceCustomer relationshipSpecial service for subscribersContact centerData science
A system for handling customer interactions with a contact center for an enterprise includes an intelligent automated agent that includes a processor, a non-transitory storage device configured to store customer profile data, and a memory. The memory has instructions stored thereon that, when executed by the processor, causes the processor to: run an artificial intelligence engine configured to learn knowledge about a customer from past interactions between the contact center and the customer, and to apply the learned knowledge to future interactions; and maintain the customer profile data on the storage device. The maintaining of the customer profile data includes retrieving the customer profile data at a beginning of a new interaction, using the retrieved customer profile data to decide how to handle the new interaction, and updating the customer profile data after completion of the new interaction to reflect the new interaction as one of the past interactions.
Owner:GENESYS TELECOMMUNICATIONS LABORATORIES INC
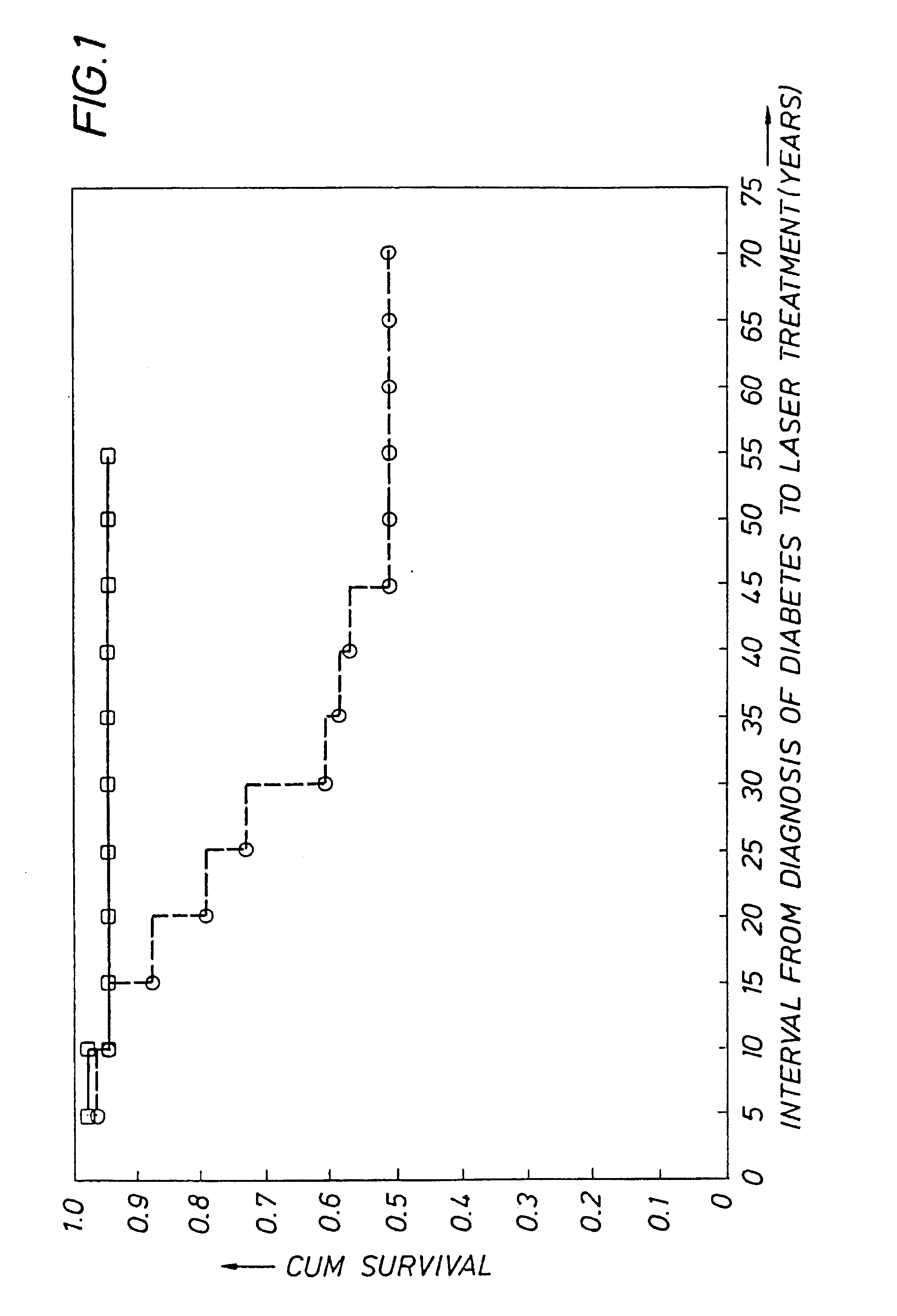
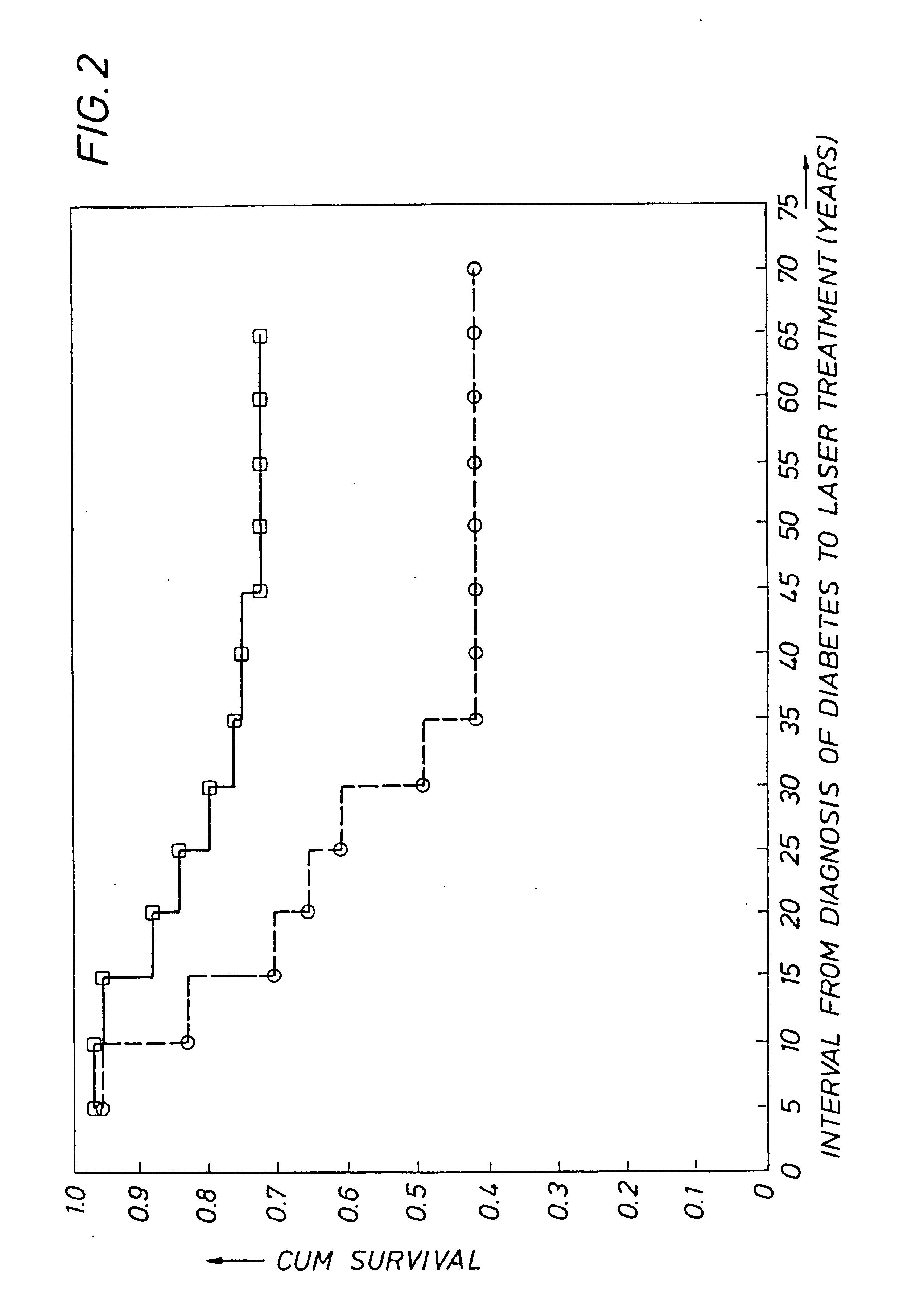
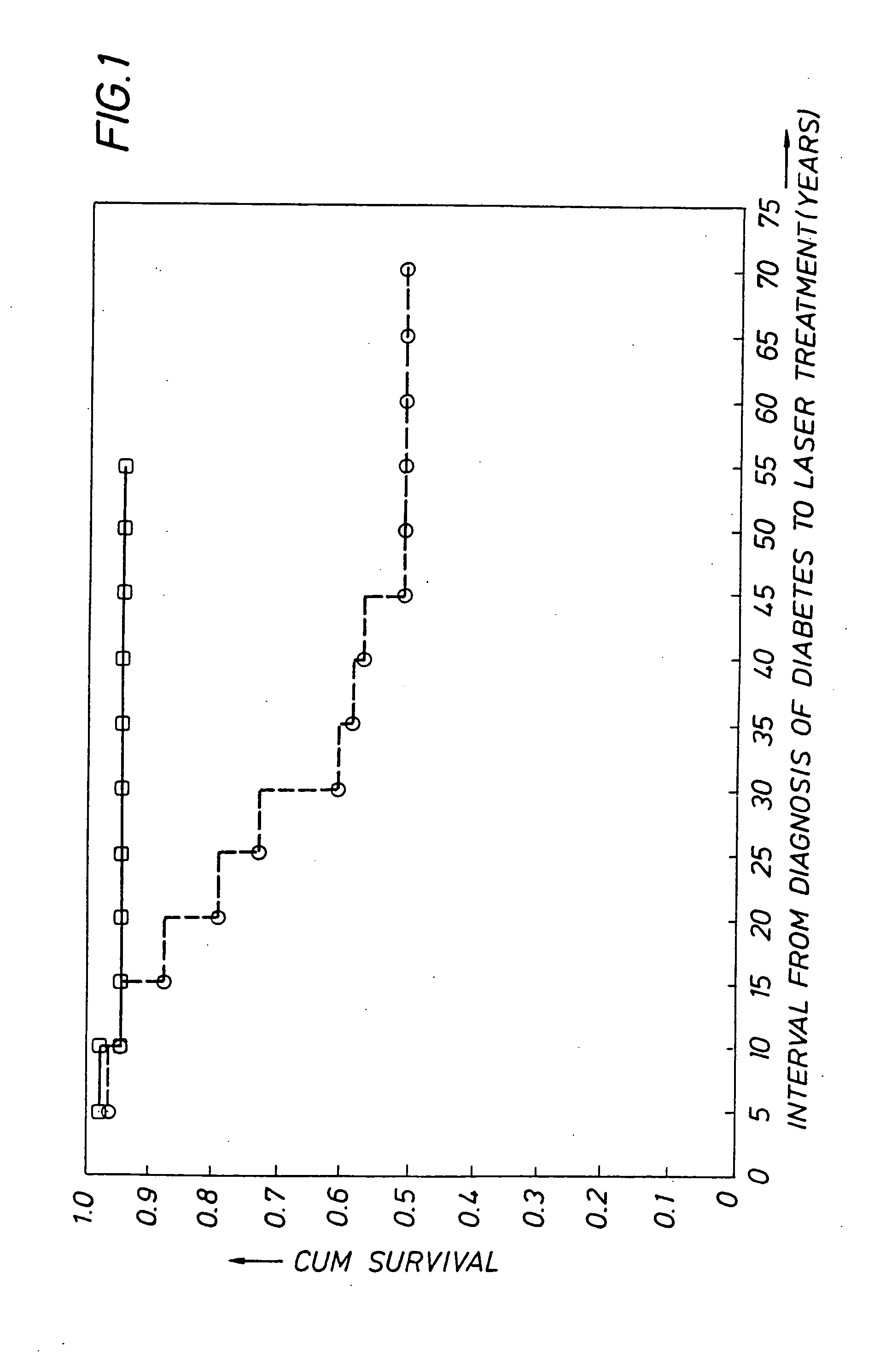
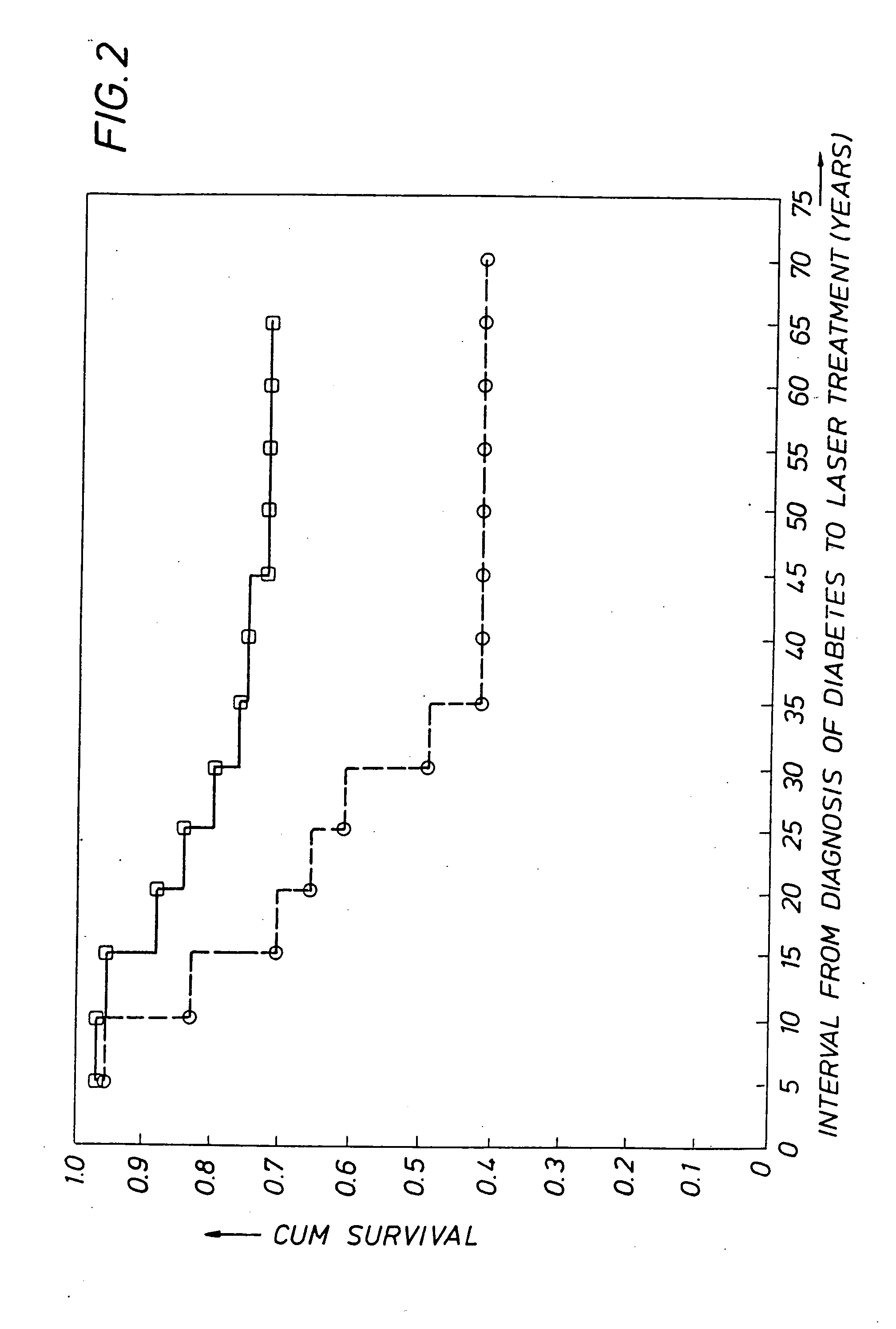
Detecting genetic predisposition to sight-threatening diabetic retinopathy
InactiveUS6713253B1Reduce and prevent occurrencePredict riskSugar derivativesMicrobiological testing/measurementDiabetes retinopathyGenomic DNA
A method and kit for predicting increased risk of sight-threatening diabetic retinopathy which includes isolating genomic DNA from a sample from a diabetic patient. The genetic polymorphism pattern for the genes IL-1A, IL-1B and IL-1RN is then identified in the DNA. The identified pattern is compared with control patterns of known polymorphisms, and patients expressing a genetic polymorphism pattern associated with increased risk of sight-threatening diabetic retinopathy are identified.
Owner:INTERLEUKIN GENETICS
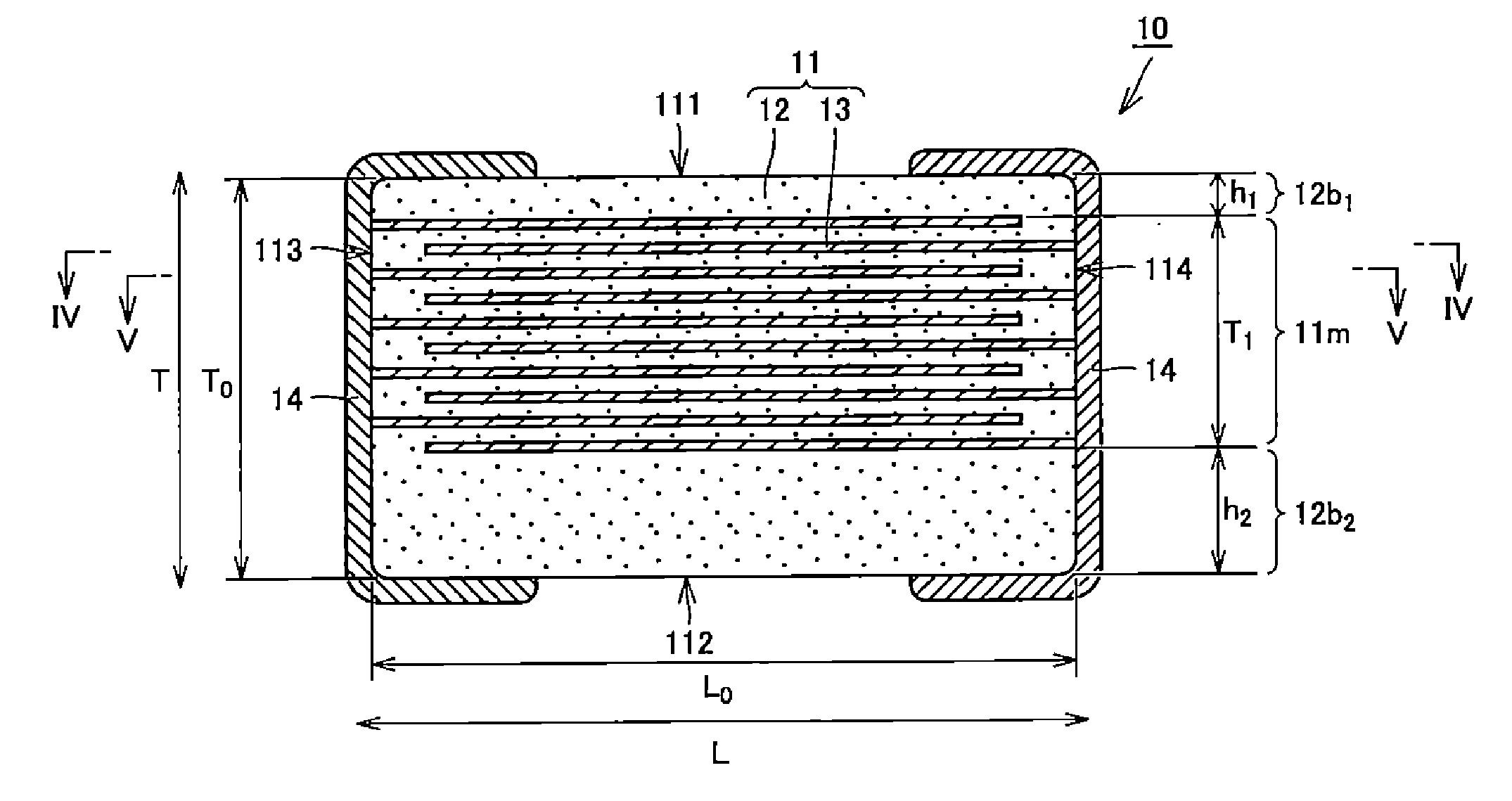
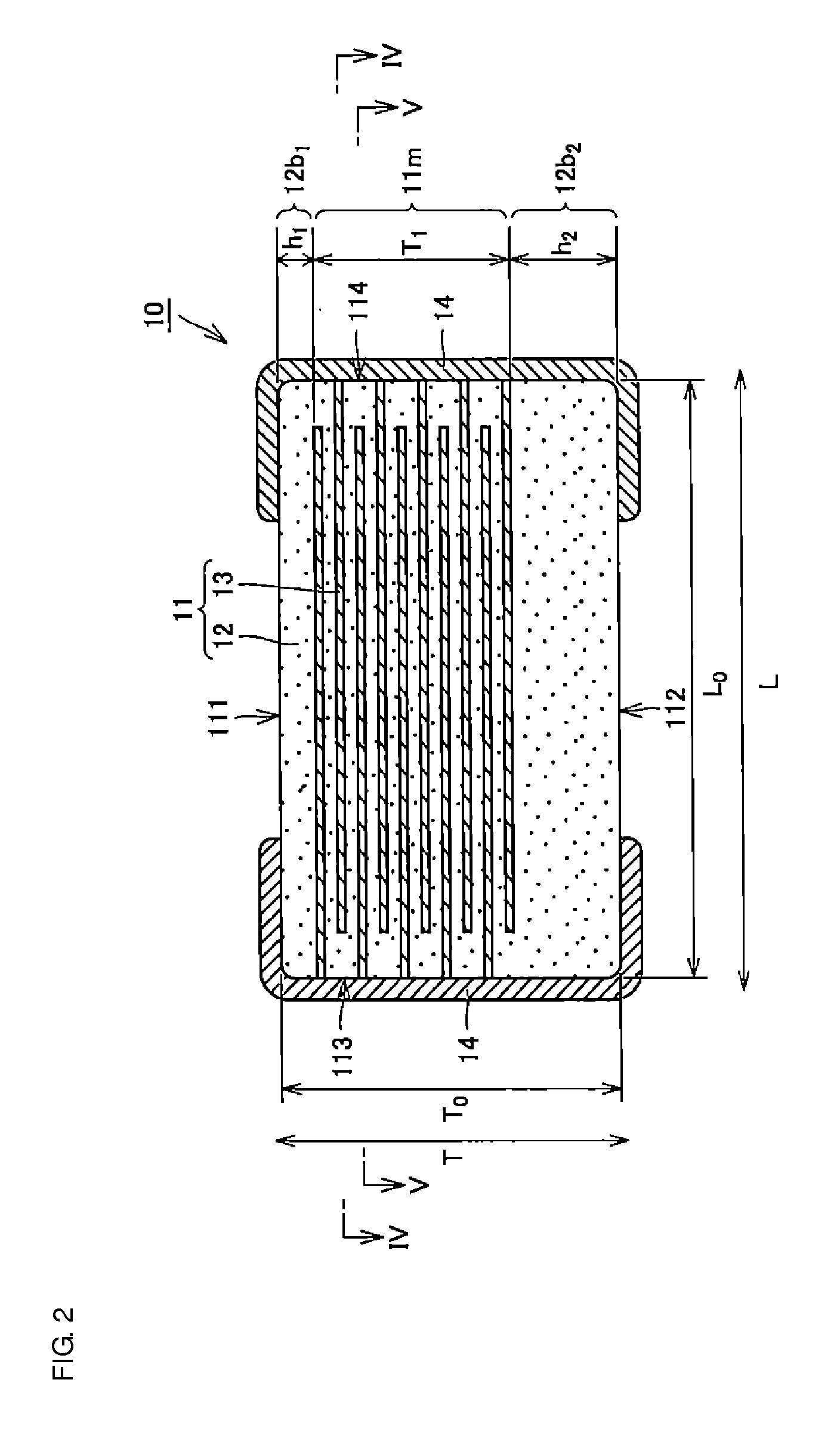
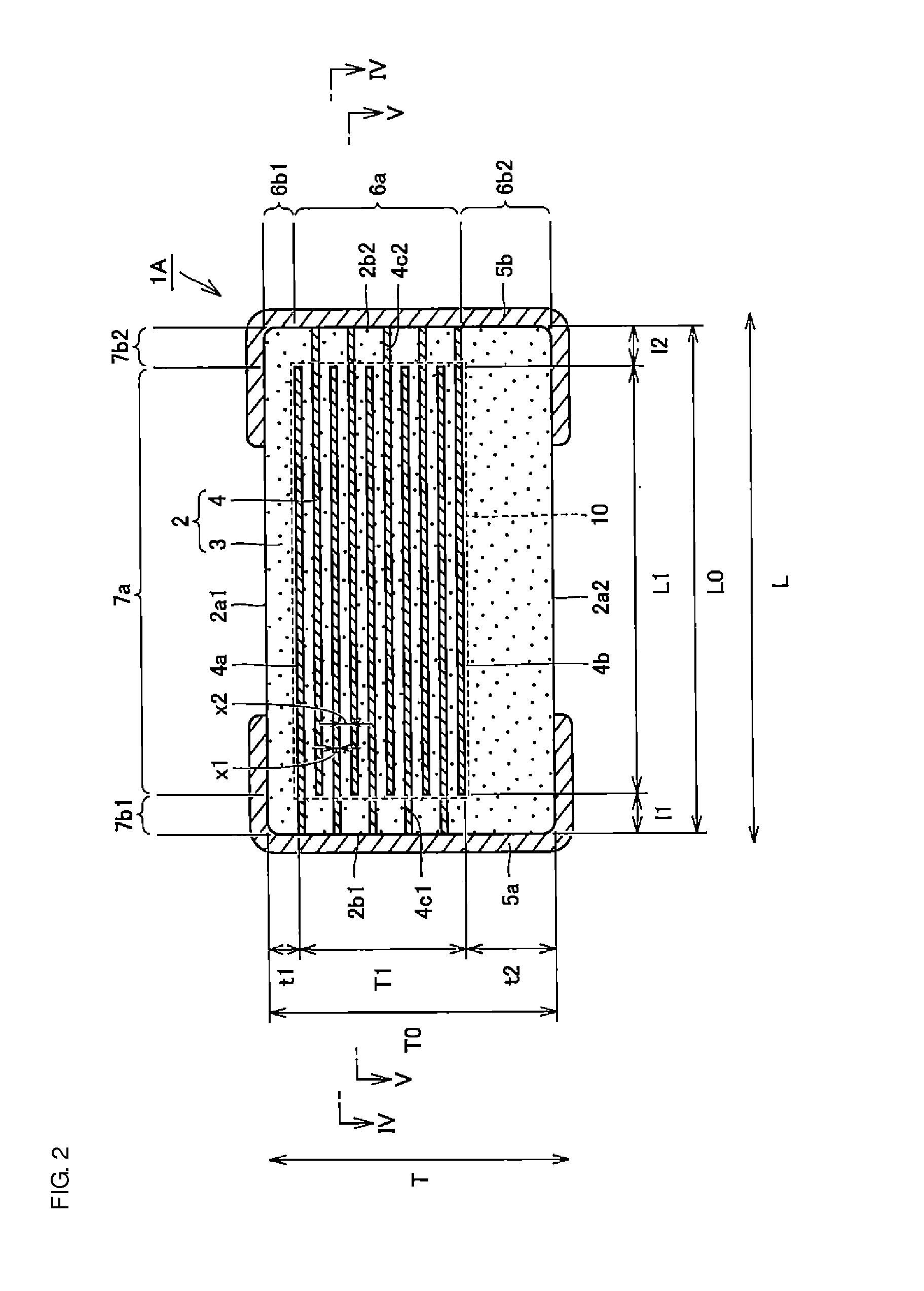
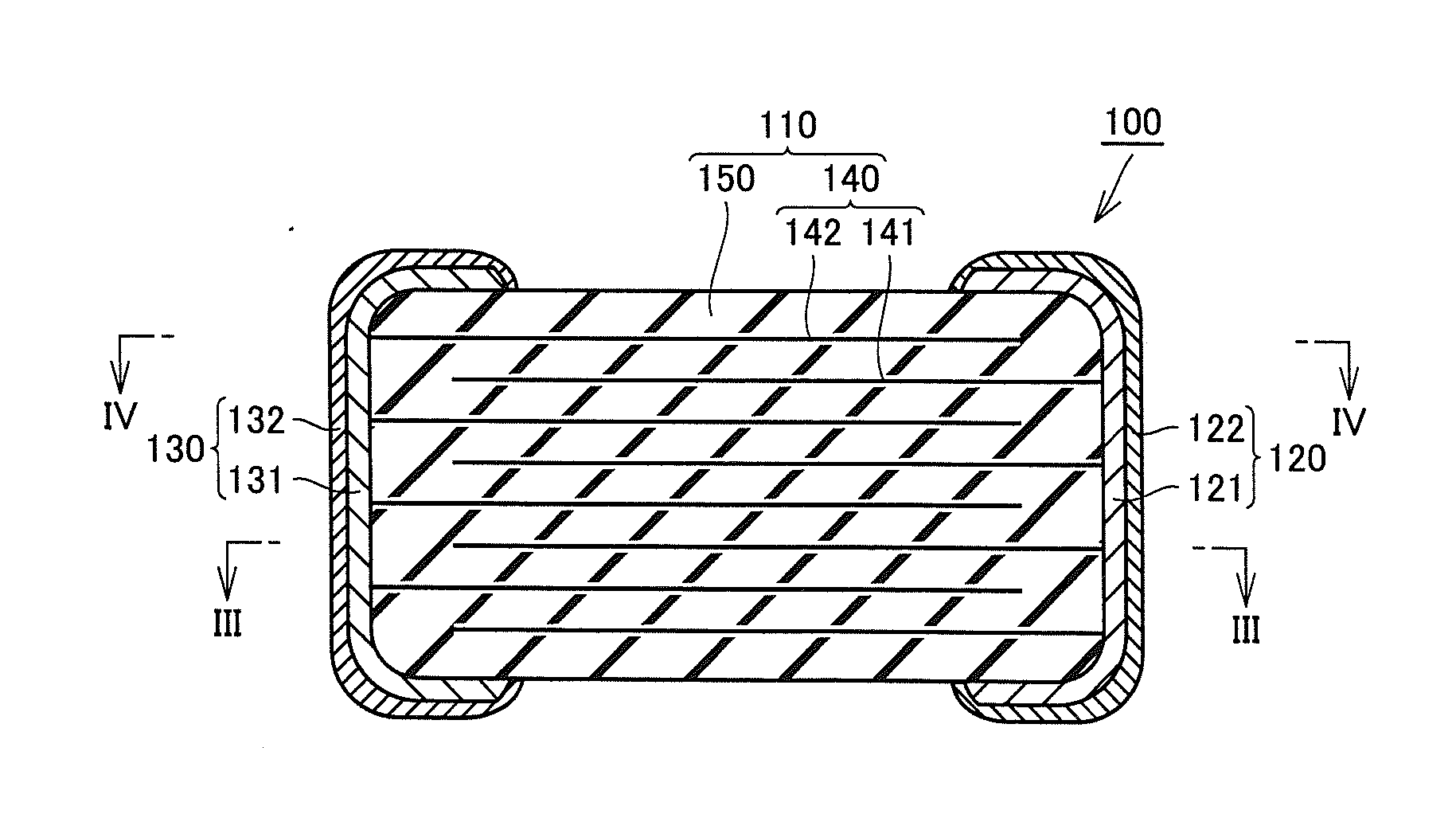
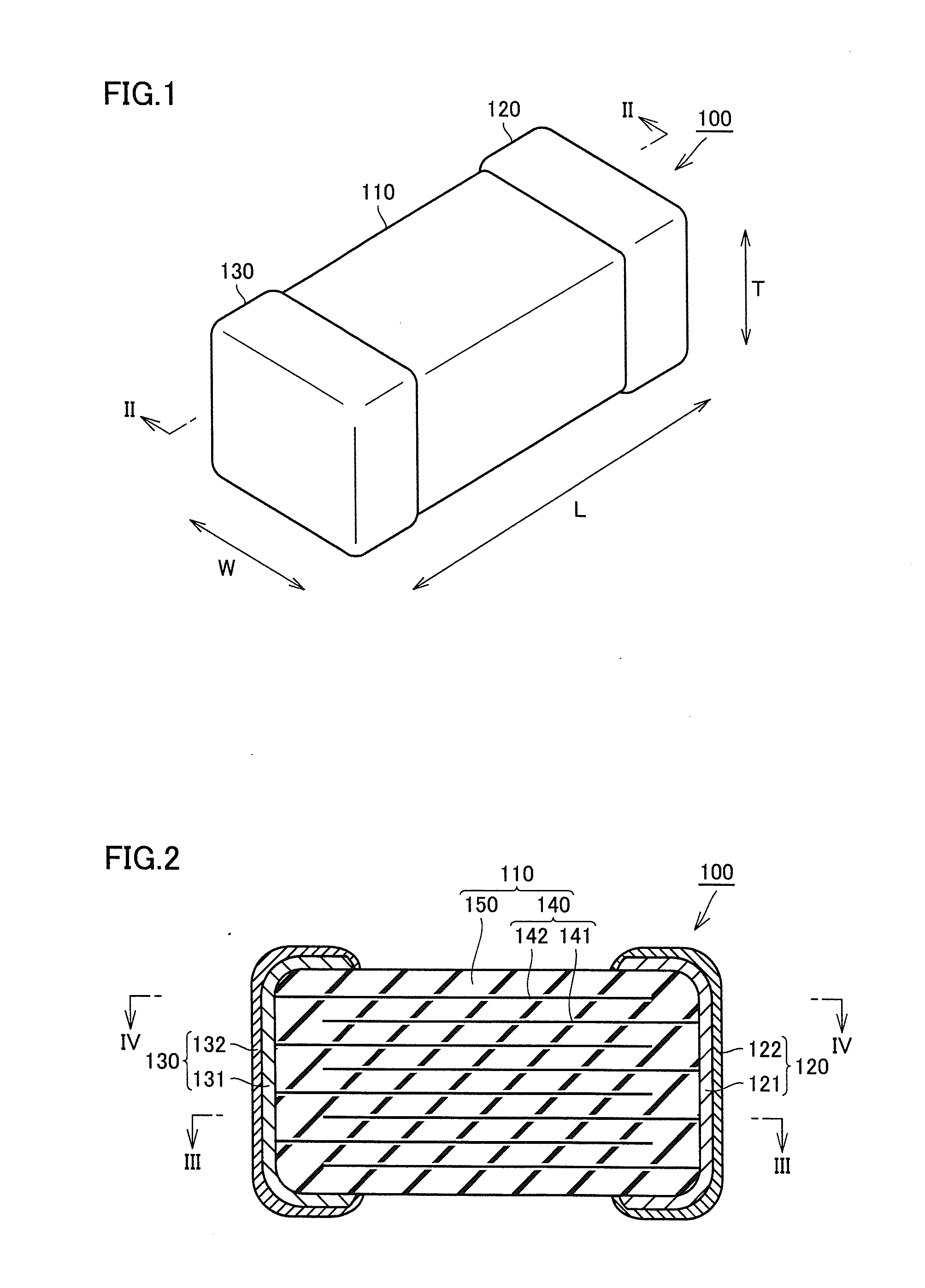
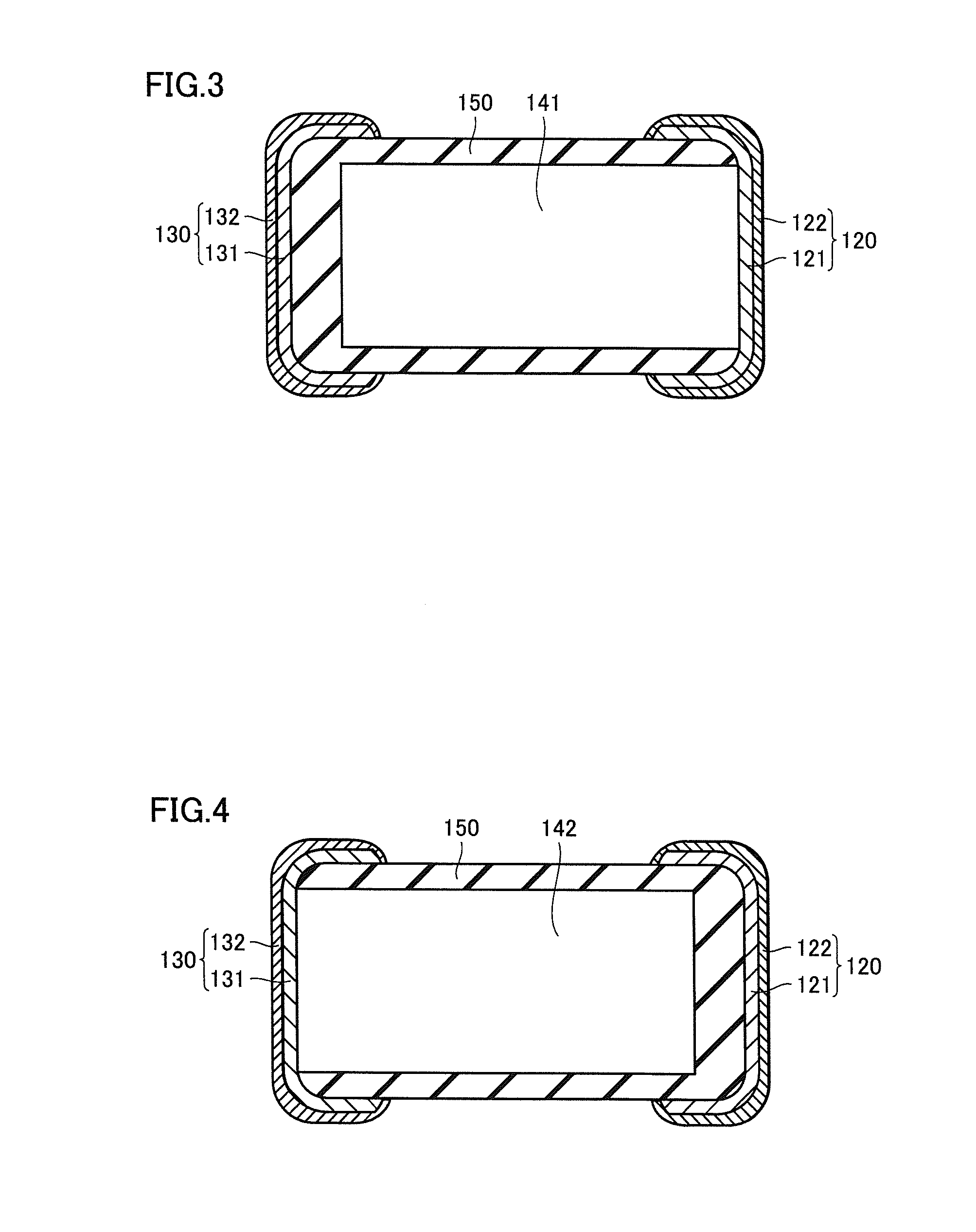
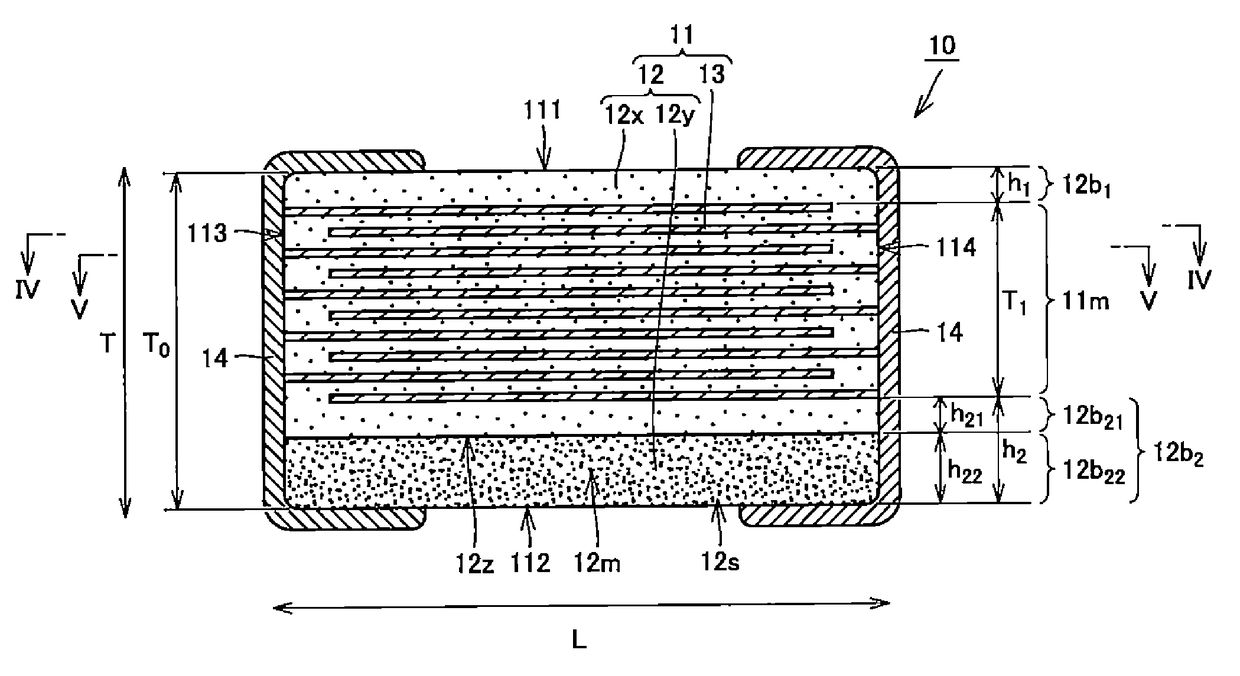
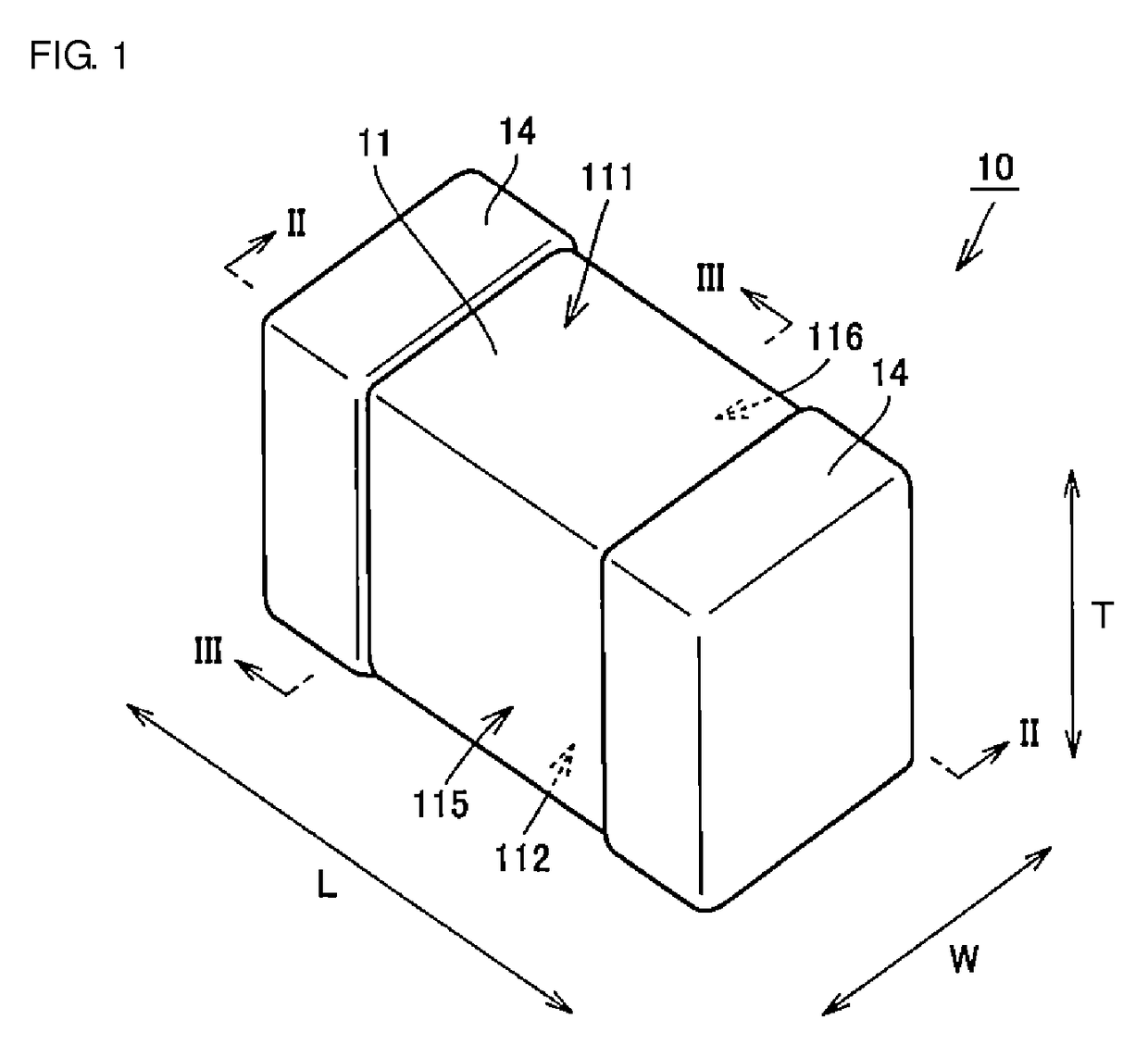
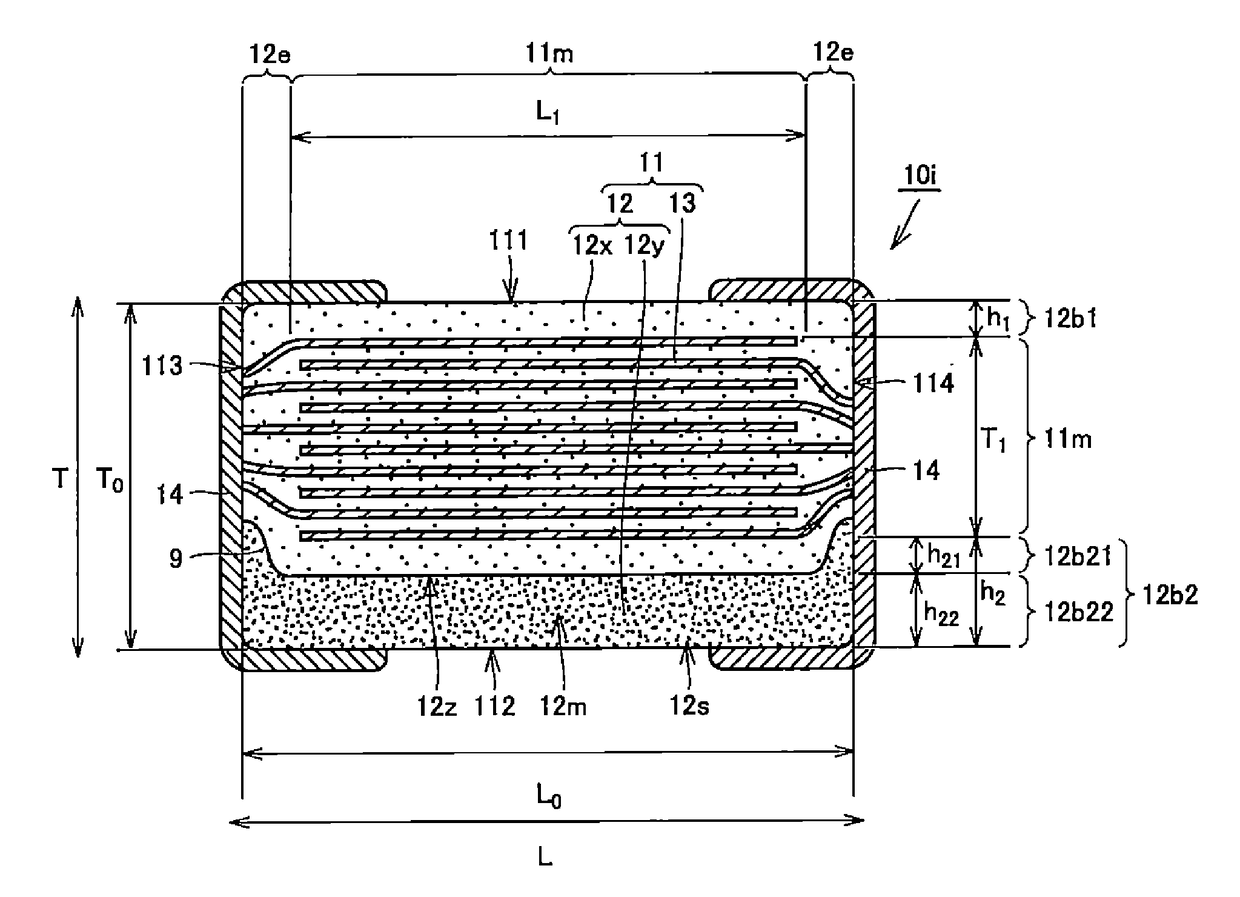
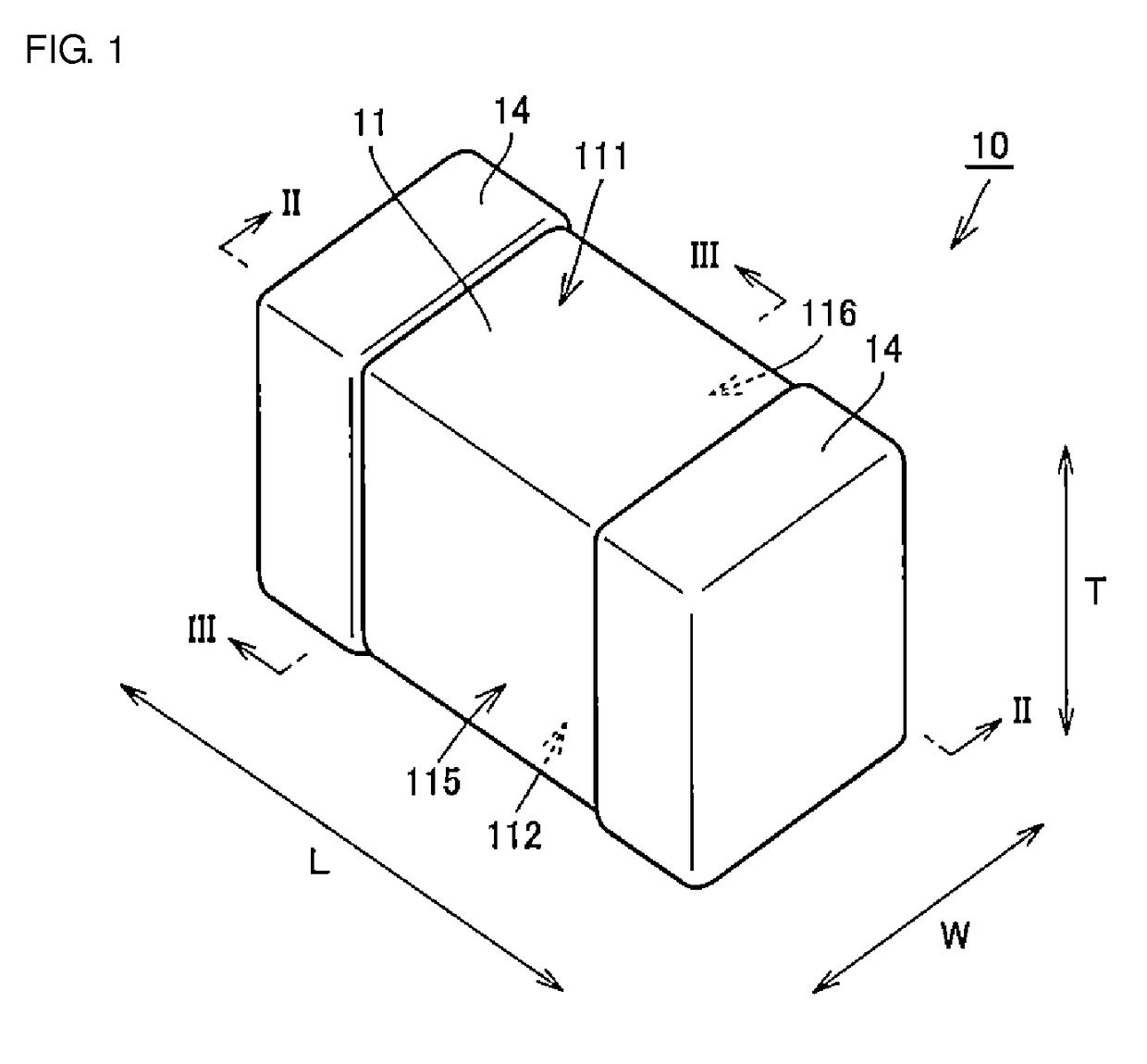
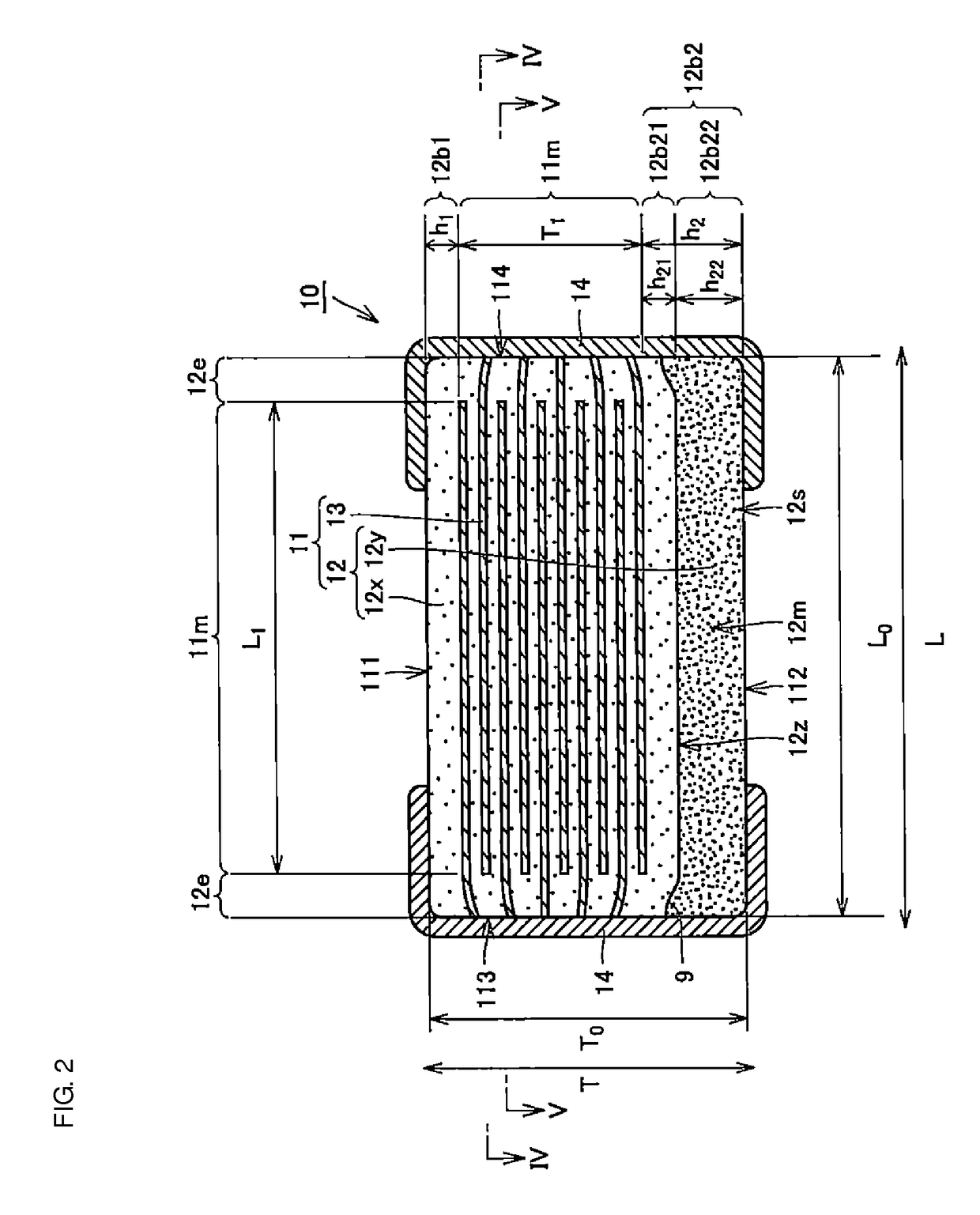
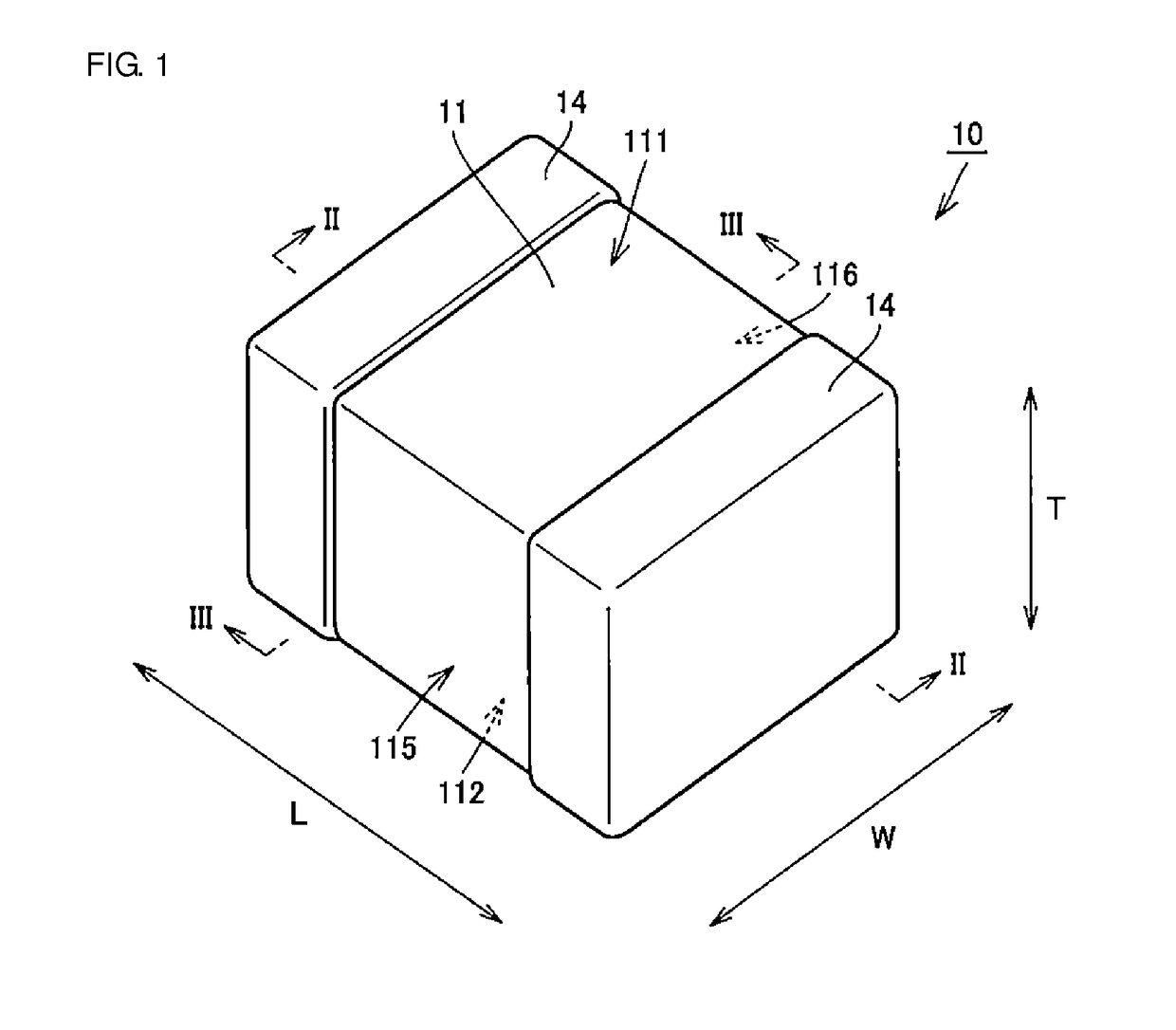
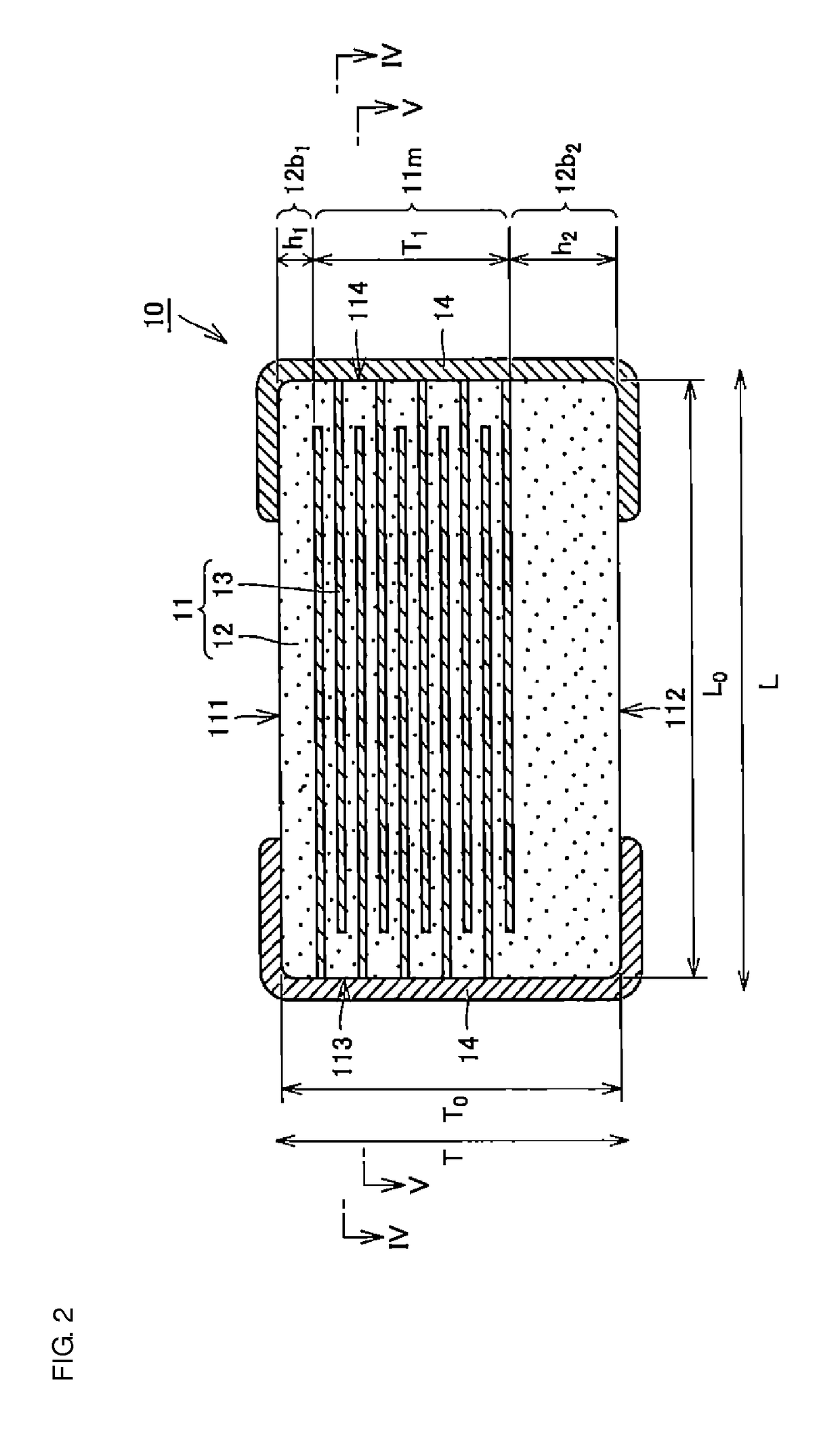
Multilayer ceramic capacitor, multilayer ceramic capacitor series including the same, and multilayer ceramic capacitor mount body including the same
ActiveUS20160049256A1Reduce and prevent occurrenceSufficient electrostatic capacitanceMultiple fixed capacitorsFixed capacitor electrodesCeramic capacitorDielectric layer
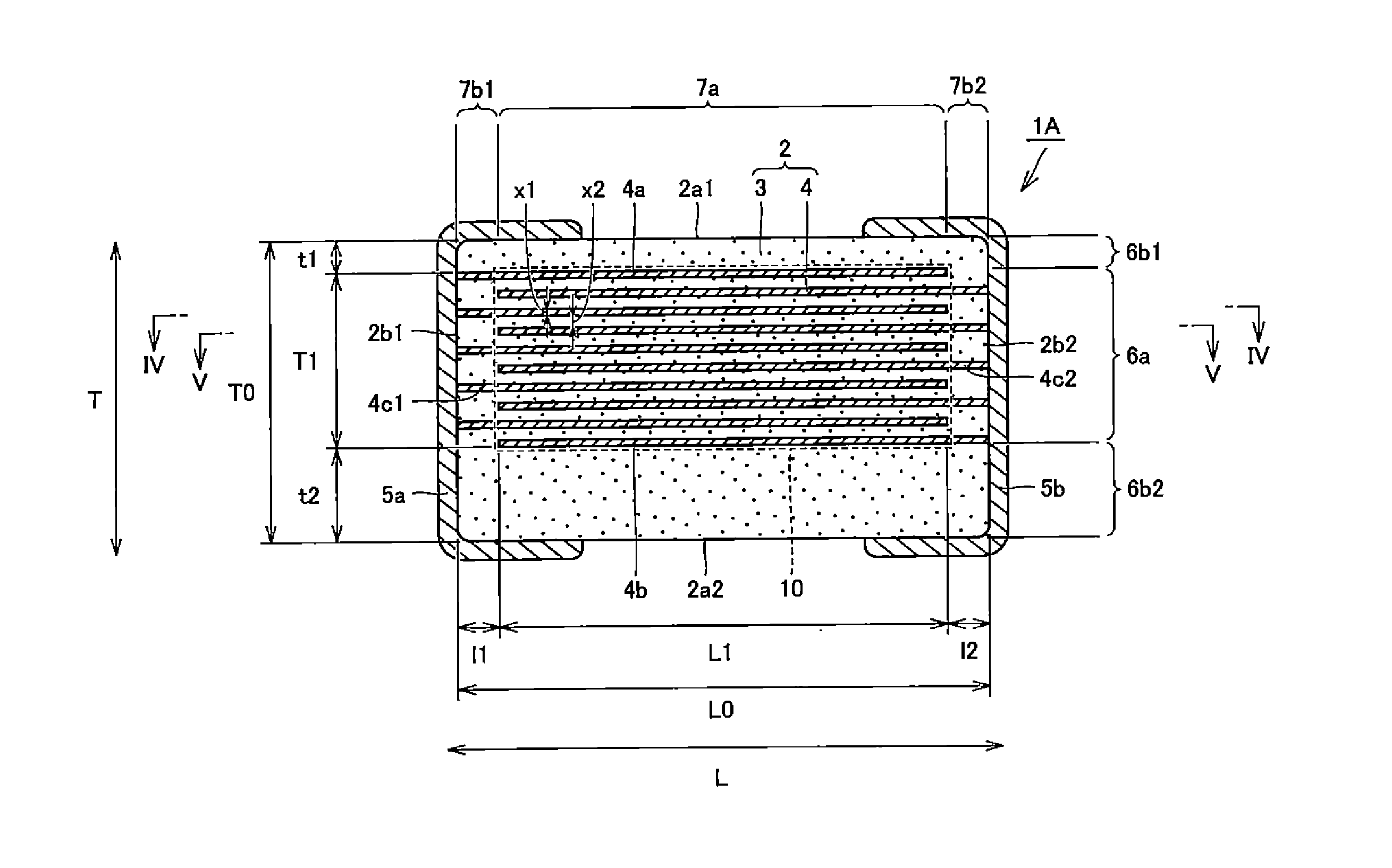
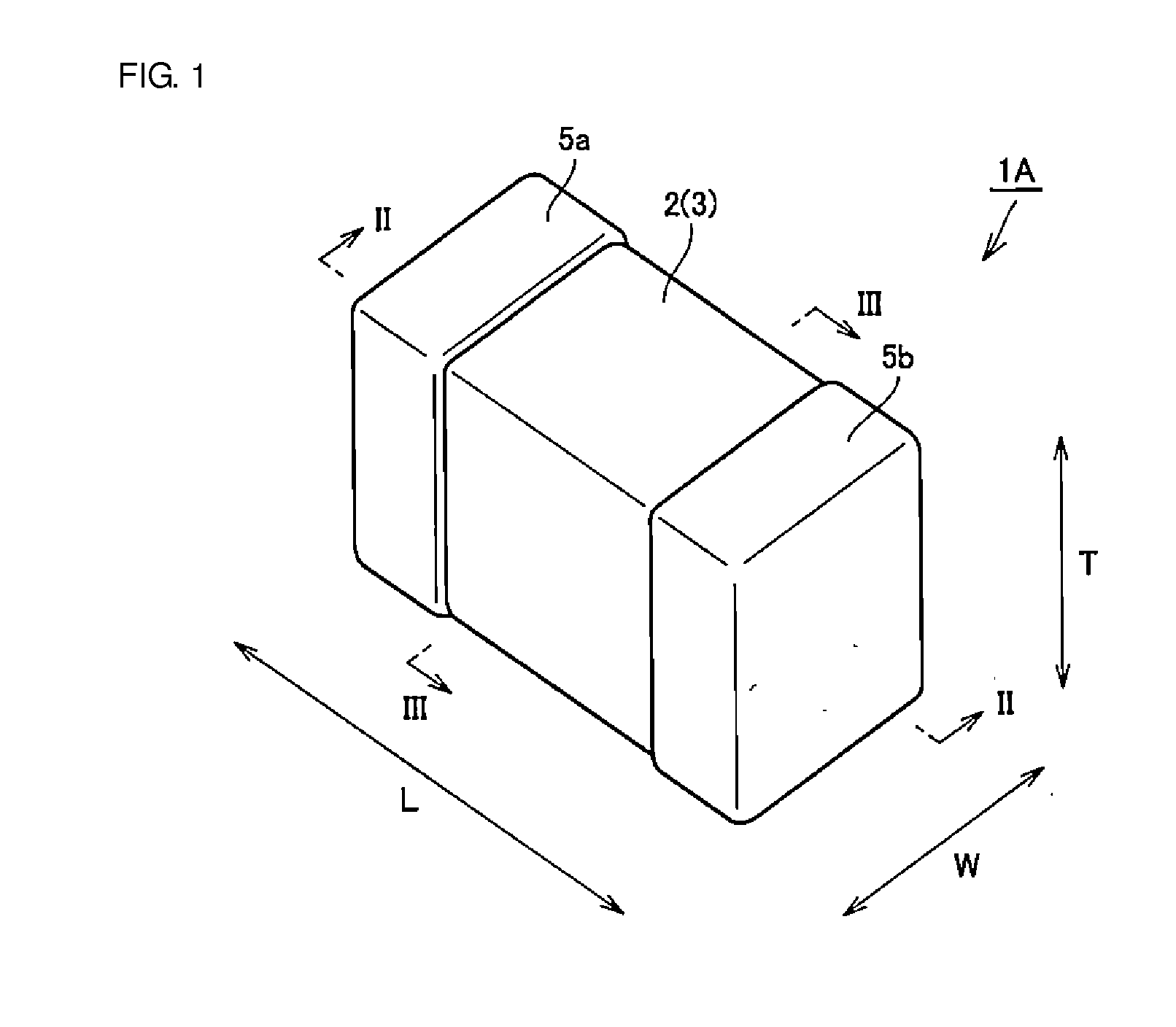
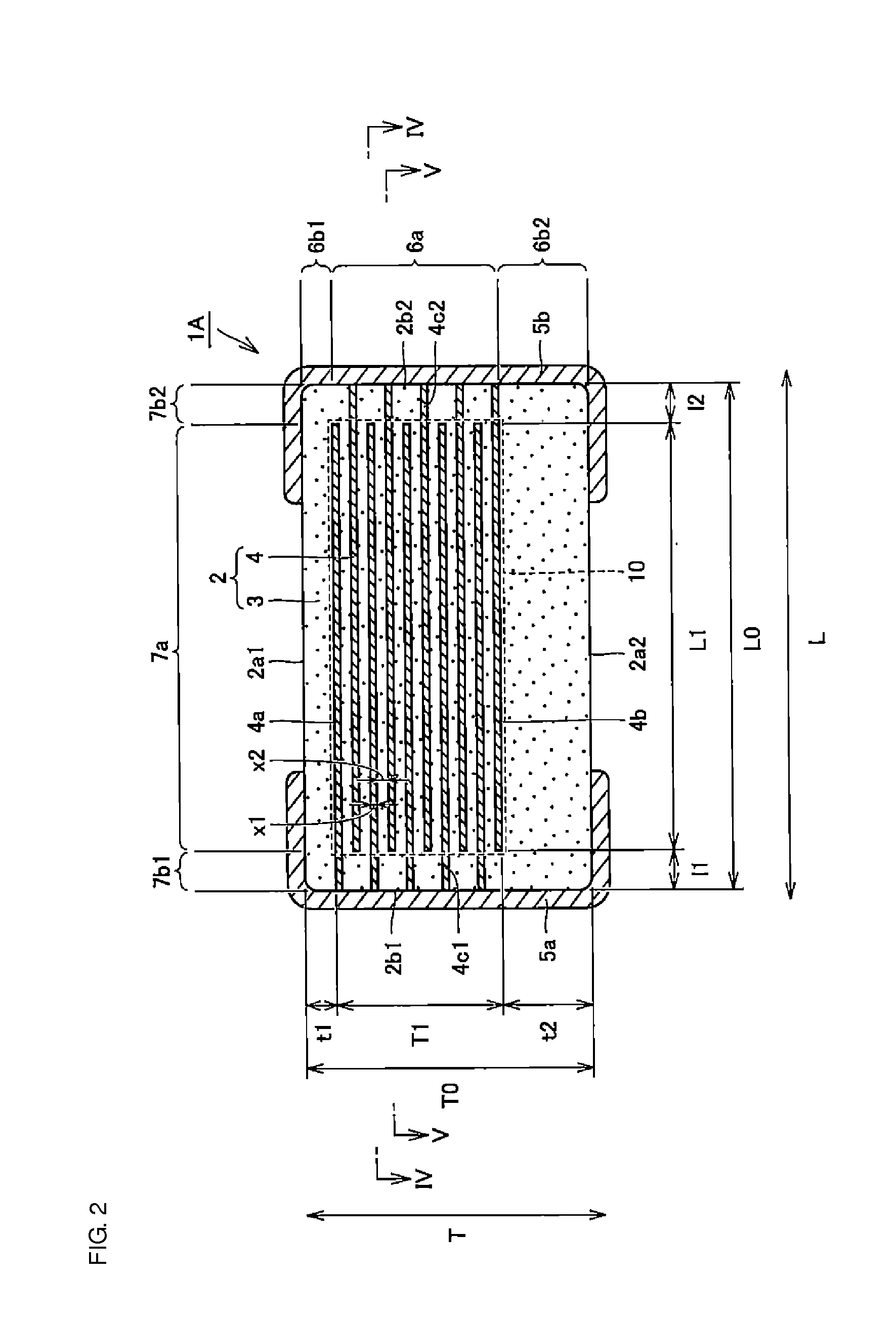
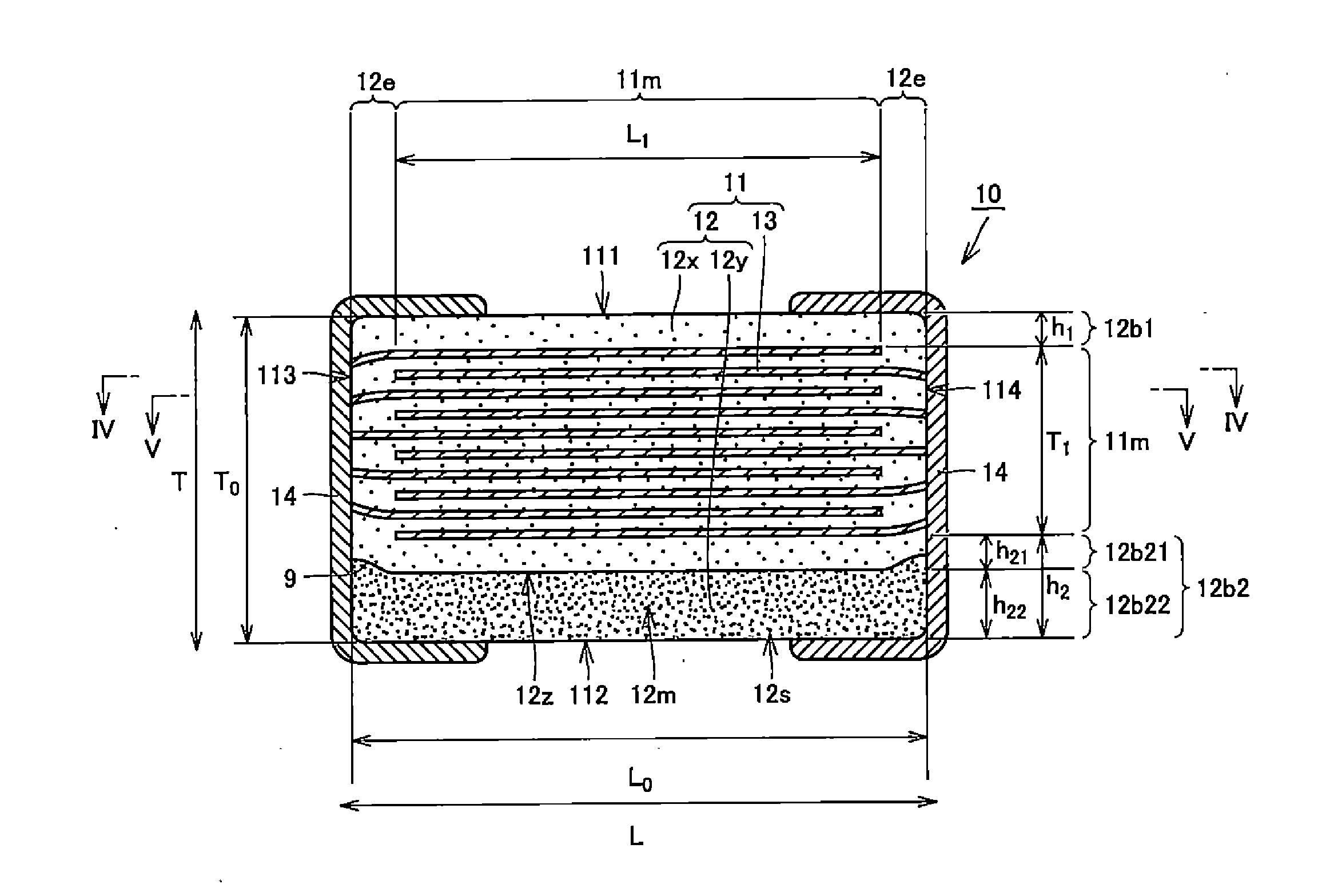
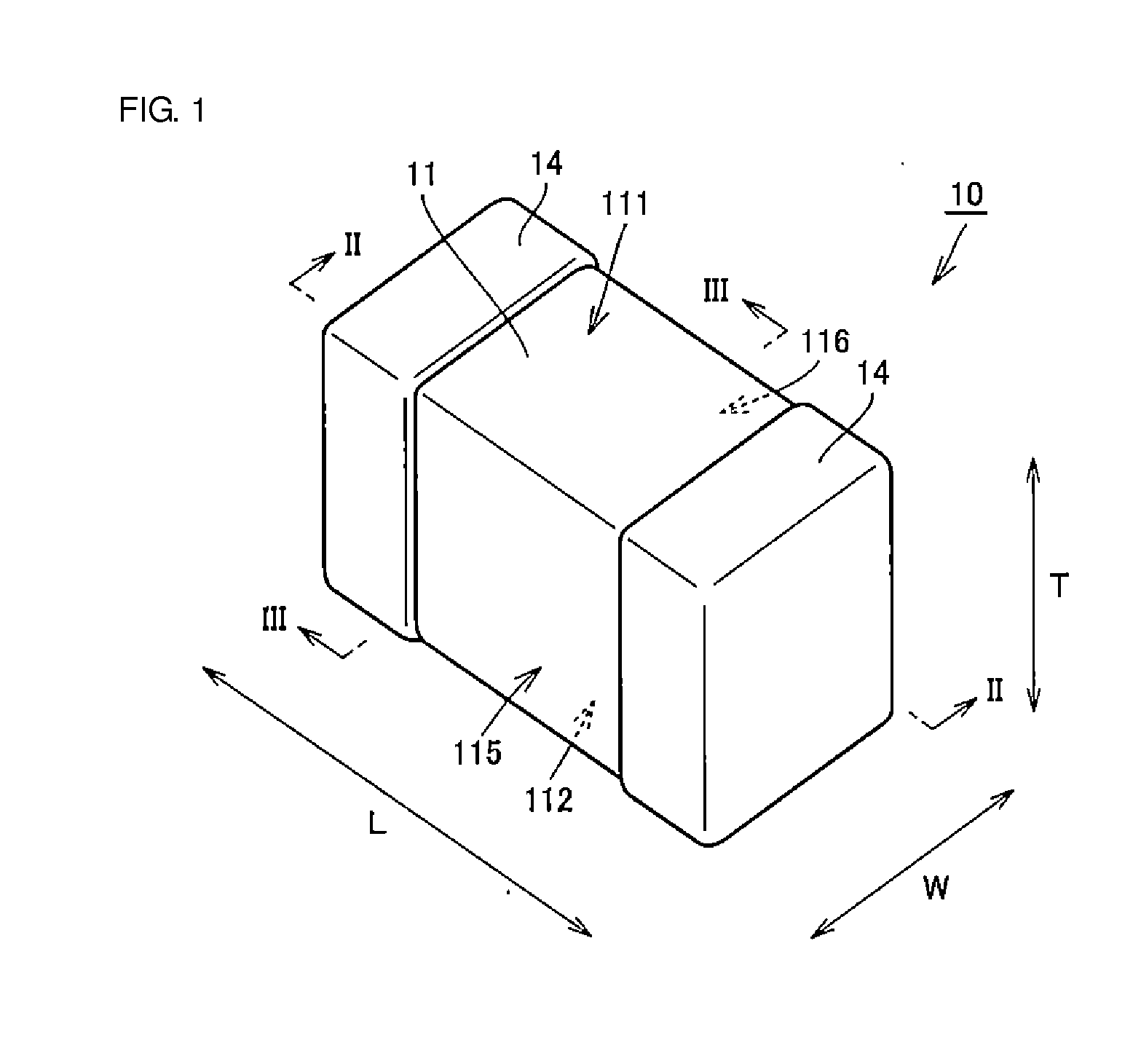
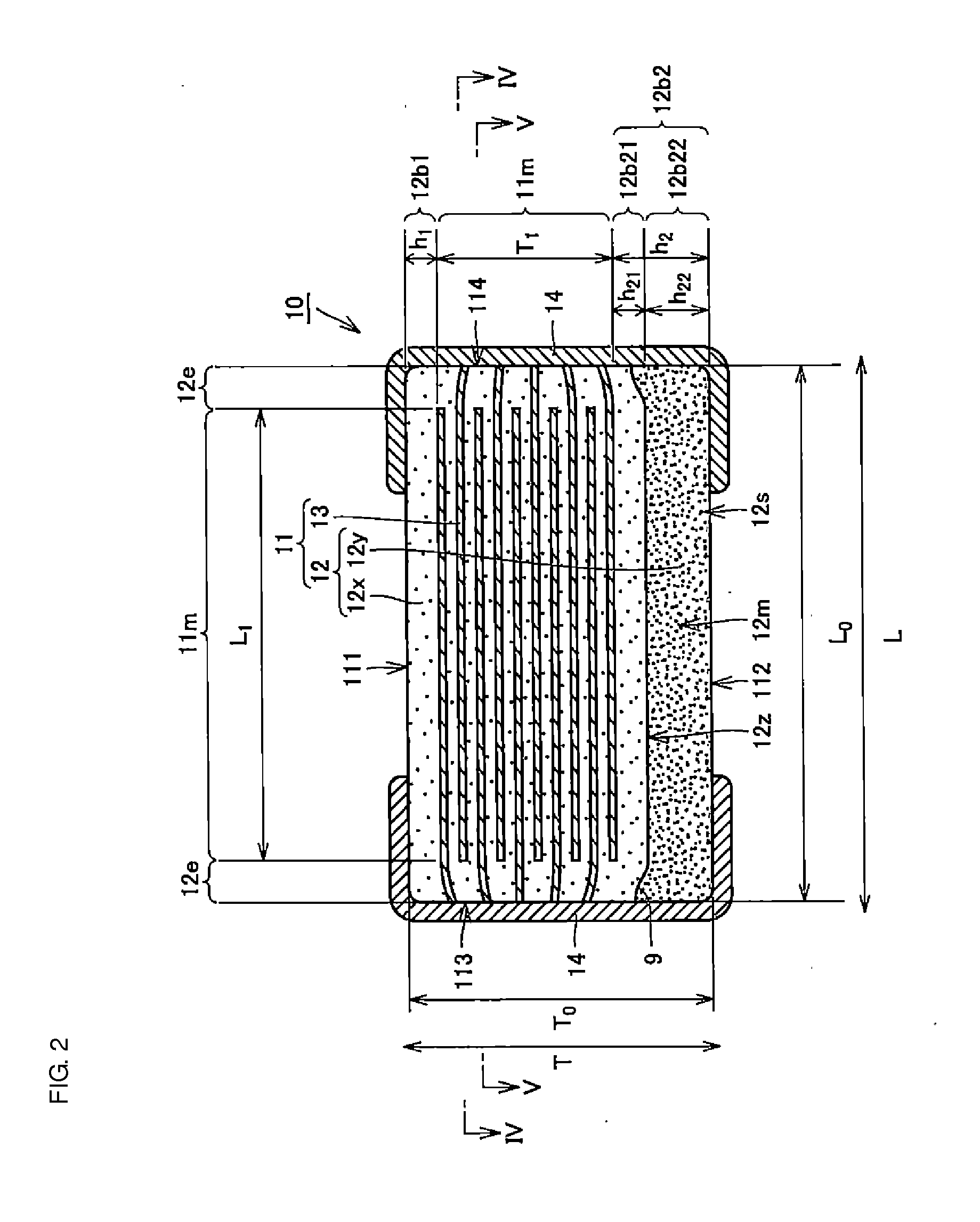
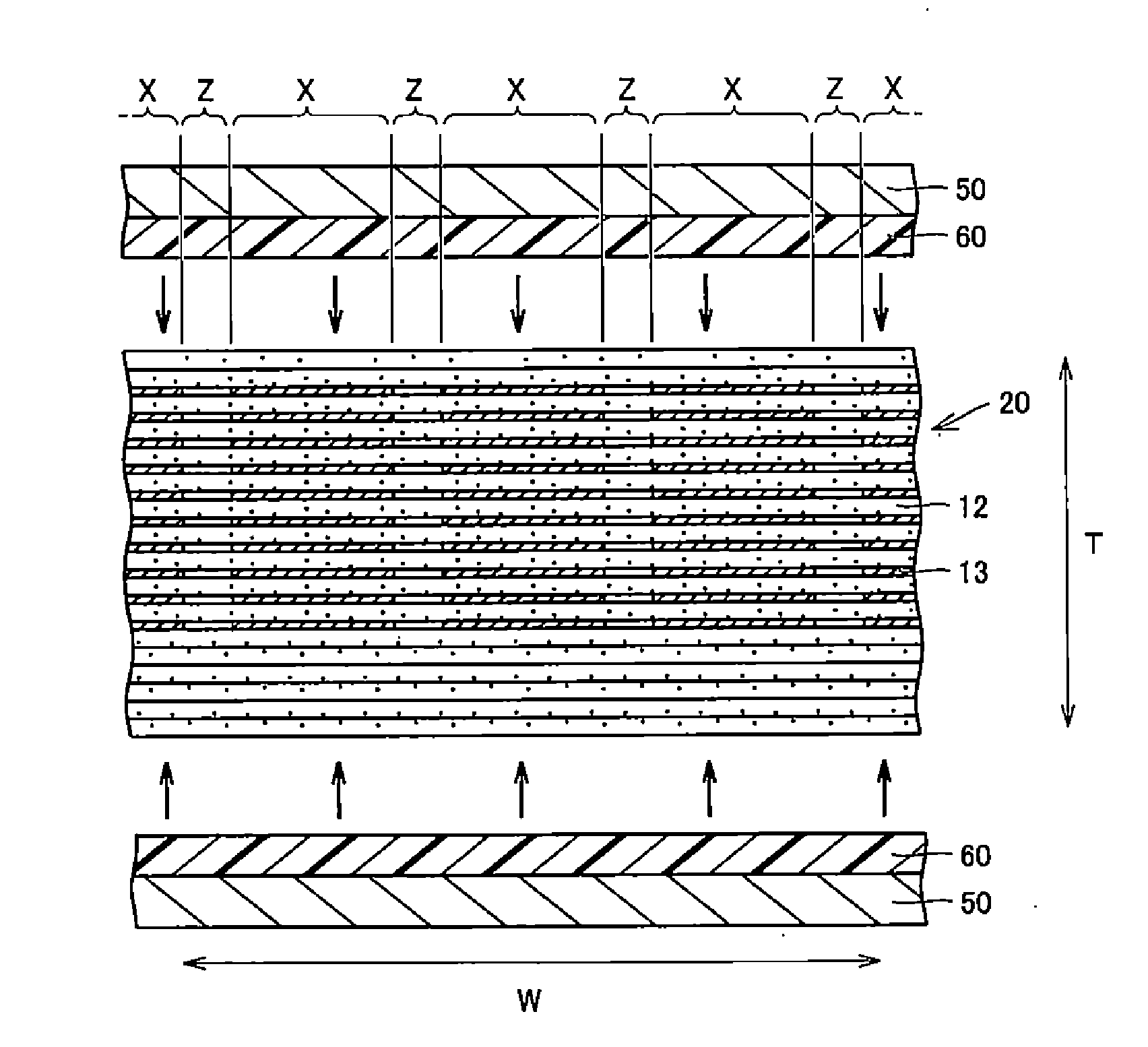
A body of a multilayer ceramic capacitor includes an inner layer portion and first and second outer layer portions sandwiching the inner layer portion therebetween. The inner layer portion includes an area extending from a conductive layer positioned closest to a first main surface to a conductive layer positioned closest to a second main surface in the stacking direction. The height of the body is smaller than the width of the body. The height of the inner layer portion is smaller than the width of the inner layer portion. The first outer layer portion includes a dielectric layer positioned closest to the first main surface. The second outer layer portion includes a dielectric layer positioned closest to the second main surface, and is thicker than the first outer layer portion. The total height of the first and second outer layer portions is smaller than the height of the inner layer portion.
Owner:MURATA MFG CO LTD
Multilayer ceramic capacitor
ActiveUS20160049244A1Reduce and prevent occurrenceFixed capacitor dielectricStacked capacitorsCeramic capacitorDielectric layer
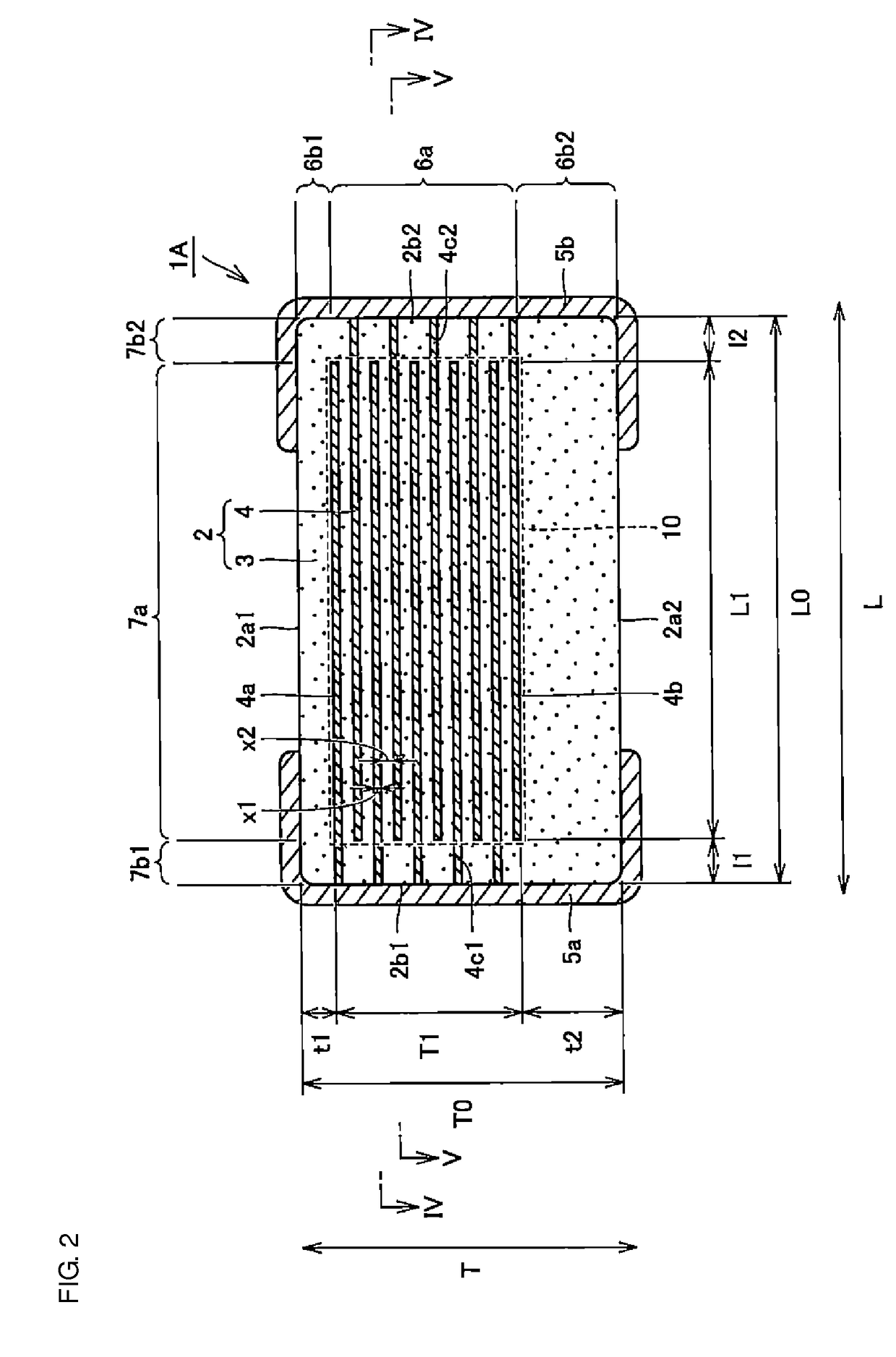
A multilayer ceramic capacitor includes a multilayer unit, thickness-direction first and second outer layer sections, and length-direction first and second outer layer sections. A dimension of the thickness-direction second outer layer section is greater than a dimension of the thickness-direction first outer layer section. The thickness-direction second outer layer section includes an inner portion and an outer portion. A composition ratio of Si to Ti in a ceramic dielectric layer included in the outer portion is higher than that in the inner portion. A Si content ratio is higher in a boundary portion between the outer portion and the inner portion. A relationship expressed by T1 / (L0−L1)≦5.98 is satisfied when a minimum dimension in the length direction of the body is denoted by L0, a minimum dimension in the thickness direction of the multilayer unit is denoted by T1, and a minimum dimension in the length direction of the multilayer unit is denoted by L1.
Owner:MURATA MFG CO LTD
Multilayer ceramic capacitor
ActiveUS20160049255A1Reduce and prevent occurrenceFixed capacitor electrodesFixed capacitor dielectricCeramic capacitorBoundary region
A multilayer ceramic capacitor includes a body and at least two outer electrodes. The body includes first and second main surfaces, an inner layer portion and first and second outer layer portions. In the inner layer portion, dielectric layers and conductive layers are alternately stacked on each other. The second outer layer portion includes an outer portion and an inner portion. A boundary region adjacent to the inner portion in the outer portion inclines toward the first main surface.
Owner:MURATA MFG CO LTD
Multilayer ceramic capacitor
ActiveUS20160049247A1Reduce and prevent occurrenceFixed capacitor electrodesFixed capacitor dielectricCeramic capacitorDielectric layer
A multilayer ceramic capacitor includes a multilayer unit, thickness-direction first and second outer layer sections, and width-direction first and second outer layer sections. A dimension of the thickness-direction second outer layer section is greater than a dimension of the thickness-direction first outer layer section. The thickness-direction second outer layer section includes an inner portion and an outer portion. A composition ratio of Si to Ti in a ceramic dielectric layer included in the outer portion is higher than that in the inner portion. A Si content ratio is higher in a boundary portion between the outer portion and the inner portion. A relationship expressed by T1 / (W0−W1)≦6.95 is satisfied when a minimum dimension in the width direction of the body is denoted by W0, a minimum dimension in the thickness direction of the multilayer unit is denoted by T1, and a minimum dimension in the width direction of the multilayer unit is denoted by W1.
Owner:MURATA MFG CO LTD
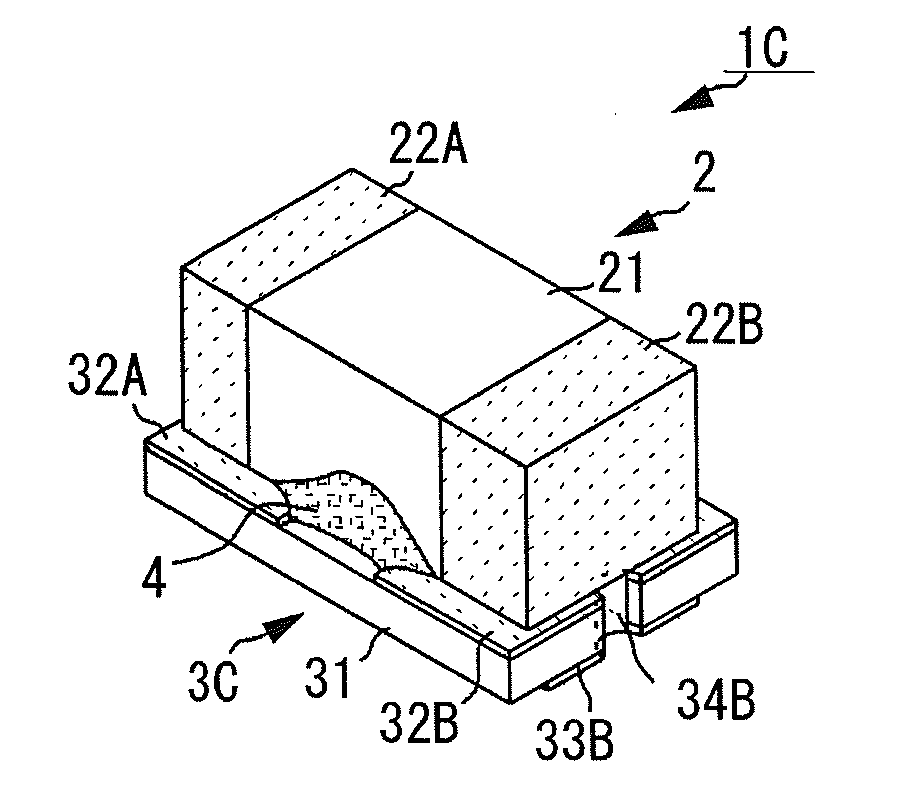
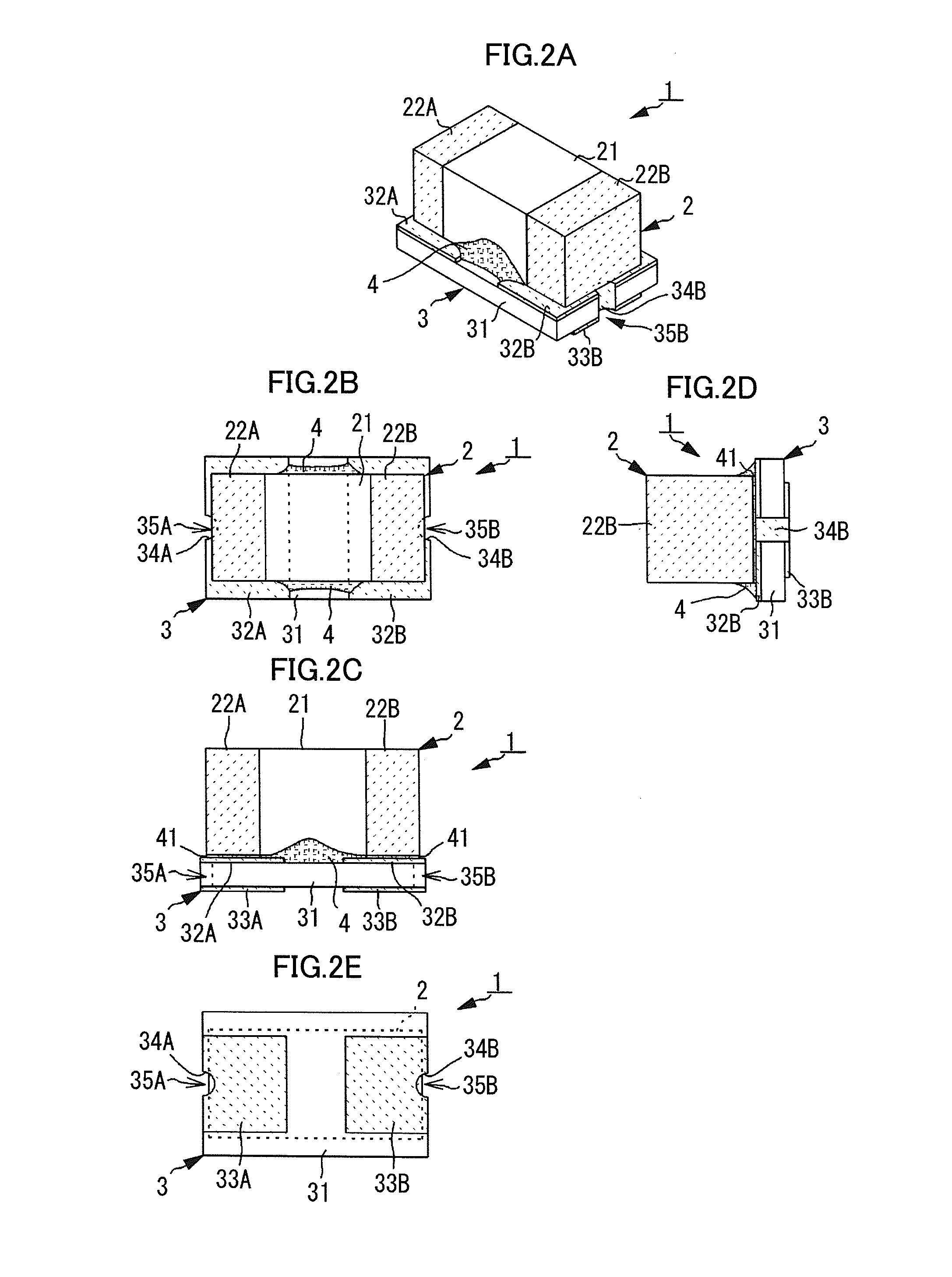
Electronic component
ActiveUS20140268486A1Reduce and prevent occurrenceReduce the amplitudeFixed capacitor housing/encapsulationStacked capacitorsSubstrate typeElectronic component
An electronic component includes a laminated capacitor and a substrate-type terminal on which the laminated capacitor is mounted, with an viscoelastic resin located in a space between the laminated capacitor and the substrate-type terminal. The substrate-type terminal includes a substrate body, component connecting electrodes to mount the laminated capacitor are located on a component mounting surface of the substrate body, and external connecting electrodes to be connected to a circuit board are located on a substrate mounting surface of the substrate body.
Owner:MURATA MFG CO LTD
Ceramic electronic component and method of manufacturing the same
ActiveUS20140375173A1Reduce and prevent occurrencePiezoelectric/electrostriction/magnetostriction machinesTransformers/inductances coils/windings/connectionsElectrical connectionAlloy
A ceramic electronic component includes a rectangular or substantially rectangular parallelepiped shaped laminate in which a ceramic layer and an internal electrode are alternately laminated and an external electrode provided on a portion of a surface of the laminate and electrically connected to the internal electrode. The external electrode includes an inner external electrode covering a portion of the surface of the laminate and including a mixture of a resin component and a metal component and an outer external electrode covering the inner external electrode and including a metal component. The inner external electrode includes, as a metal component, a first metal component of which a portion forms an alloy with the internal electrode so as to connect the internal electrode and the inner external electrode to each other, and a second metal component higher in melting point than the first metal component, of which a portion forms an alloy with the first metal component so as to connect the inner external electrode and the outer external electrode to each other. A concentration of a metal in a surface layer of the inner external electrode is not lower than about 17%.
Owner:MURATA MFG CO LTD
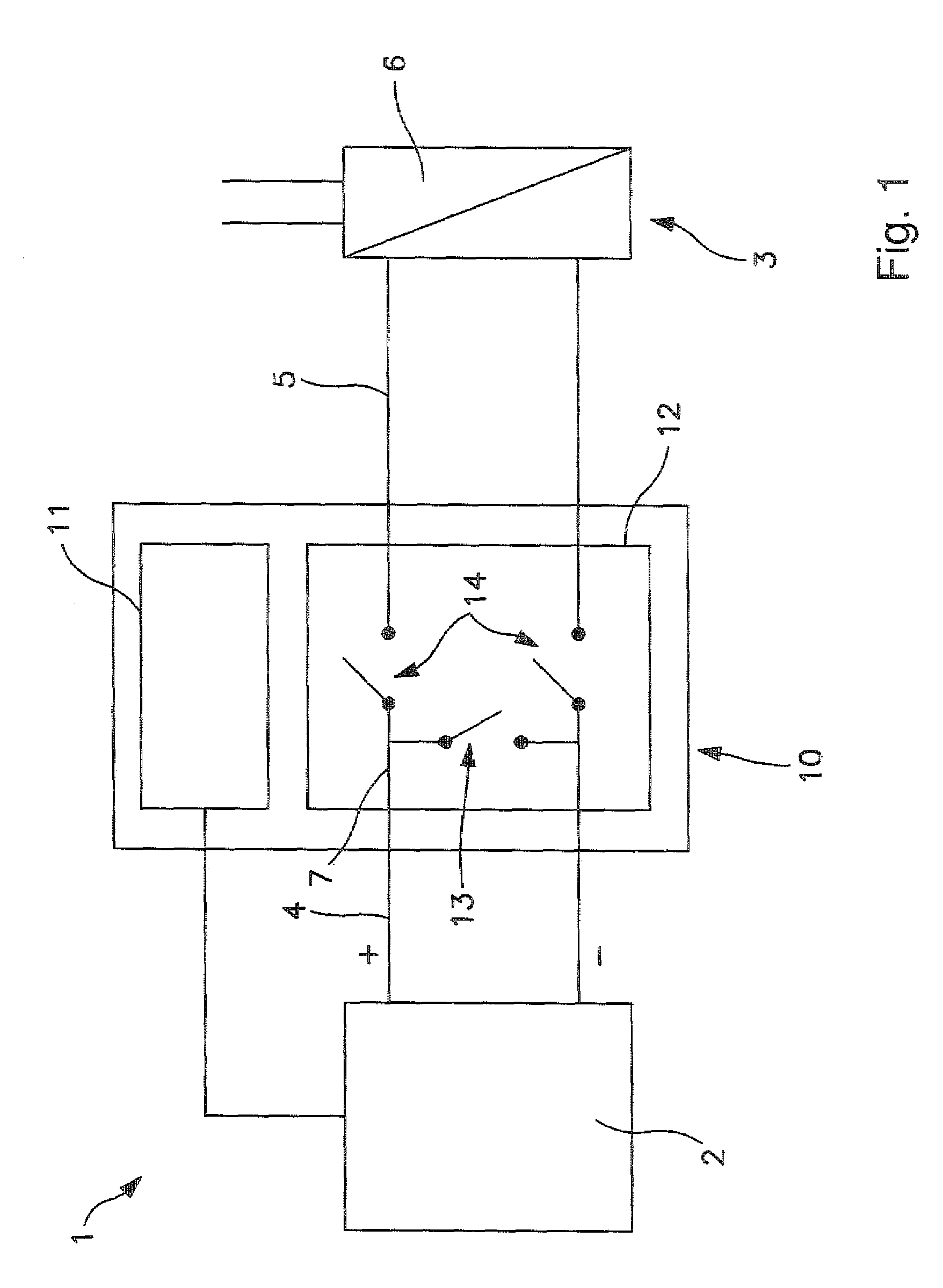
Method and device for improving the service life of a fuel cell at transitions in operation
ActiveUS20090263683A1Prevent degradationExtended service lifeFuel cell auxillariesCell component detailsFuel cellsCombustion chamber
The subject of the present invention relates to a method and a protector for reducing degradation of fuel cell systems at transitions in operation, in particular at electrodes or catalysts in a combustion chamber of a stack of a PEM fuel cell system in startup and shutoff events of the fuel cell system. A switchable material delivery device is provided for varying a delivery of material to the fuel cell system, so that a transition from a first state of the fuel cell system to a second state of the fuel cell system can be initiated, such that a potential difference between different electrodes can be effected. At least one reducing mechanism is provided for reducing the potential difference between the different electrodes during the transition, in which the reducing mechanism includes at least one compensating device for an unequal gas distribution by reducing the proportions causing degradation, to reduce degradation. The compensation device includes at least one short-circuiting unit, with which the different electrodes can be short-circuited, in order to reduce the potential difference.
Owner:ROBERT BOSCH GMBH
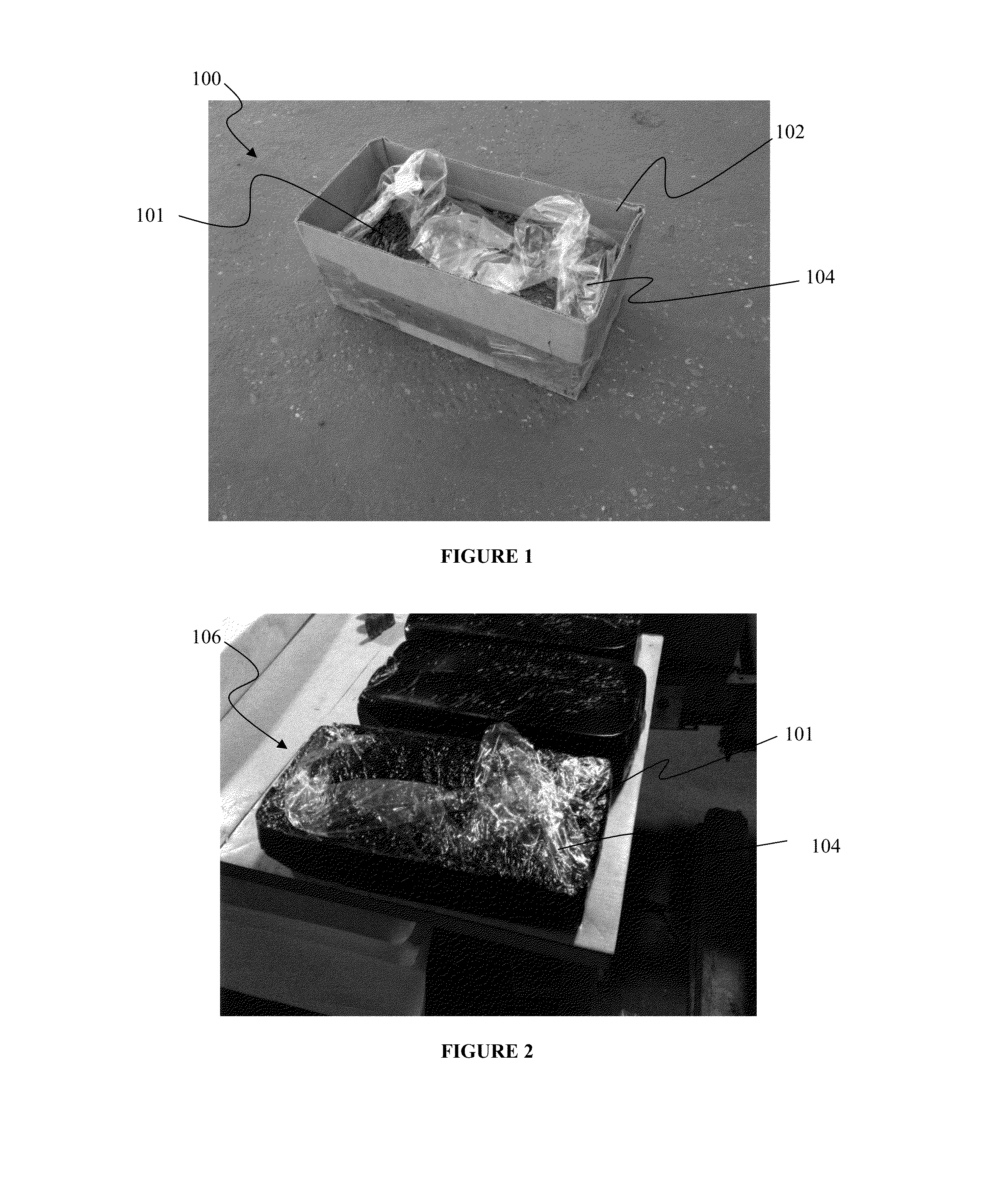
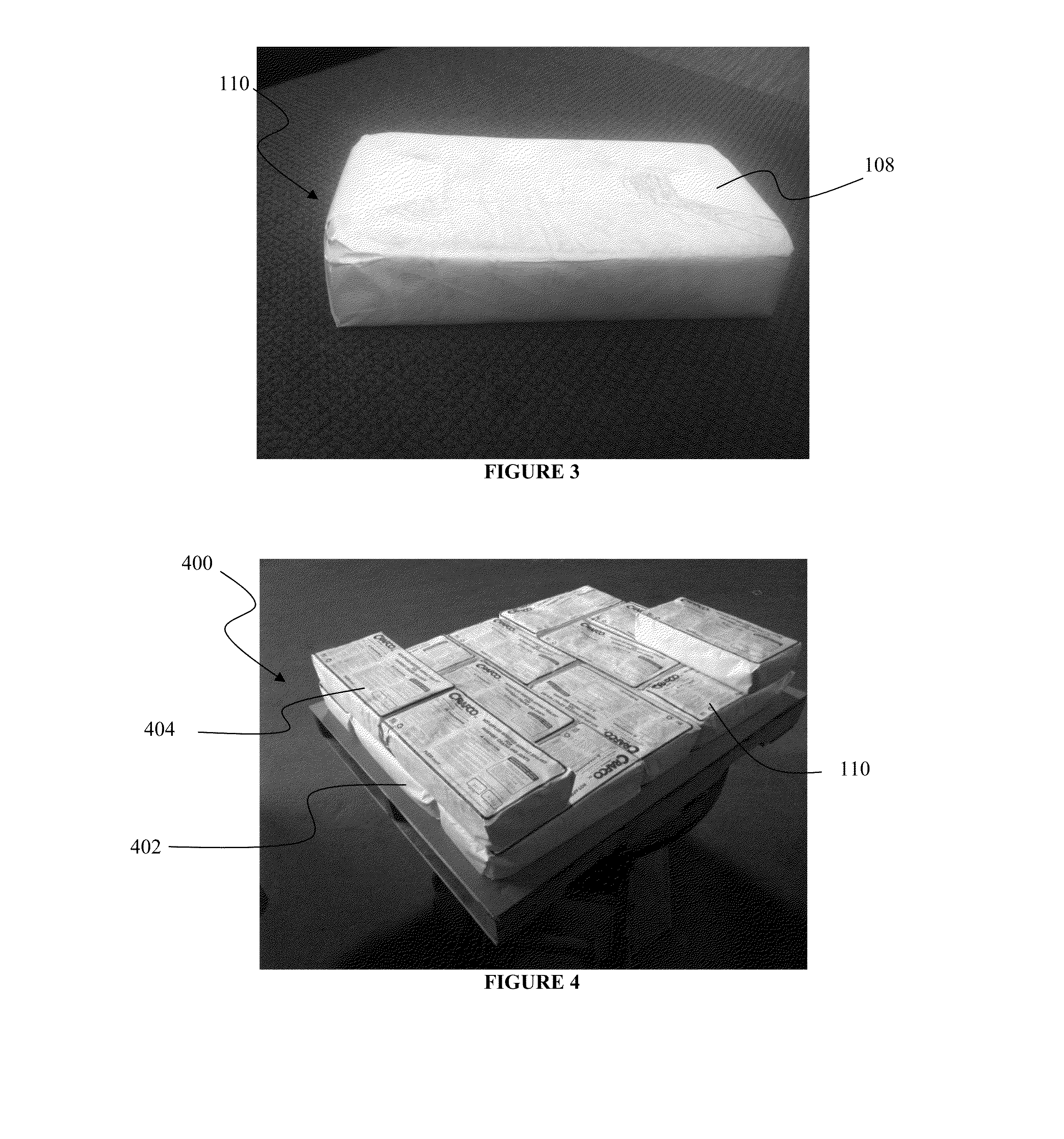
Durable, consumable packaging system for hot melt materials and methods of making and using same
ActiveUS20130075298A1Superior moisture resistanceHigh opacityFlexible coversWrappersHigh-density polyethyleneBiomedical engineering
Owner:CRAFCO
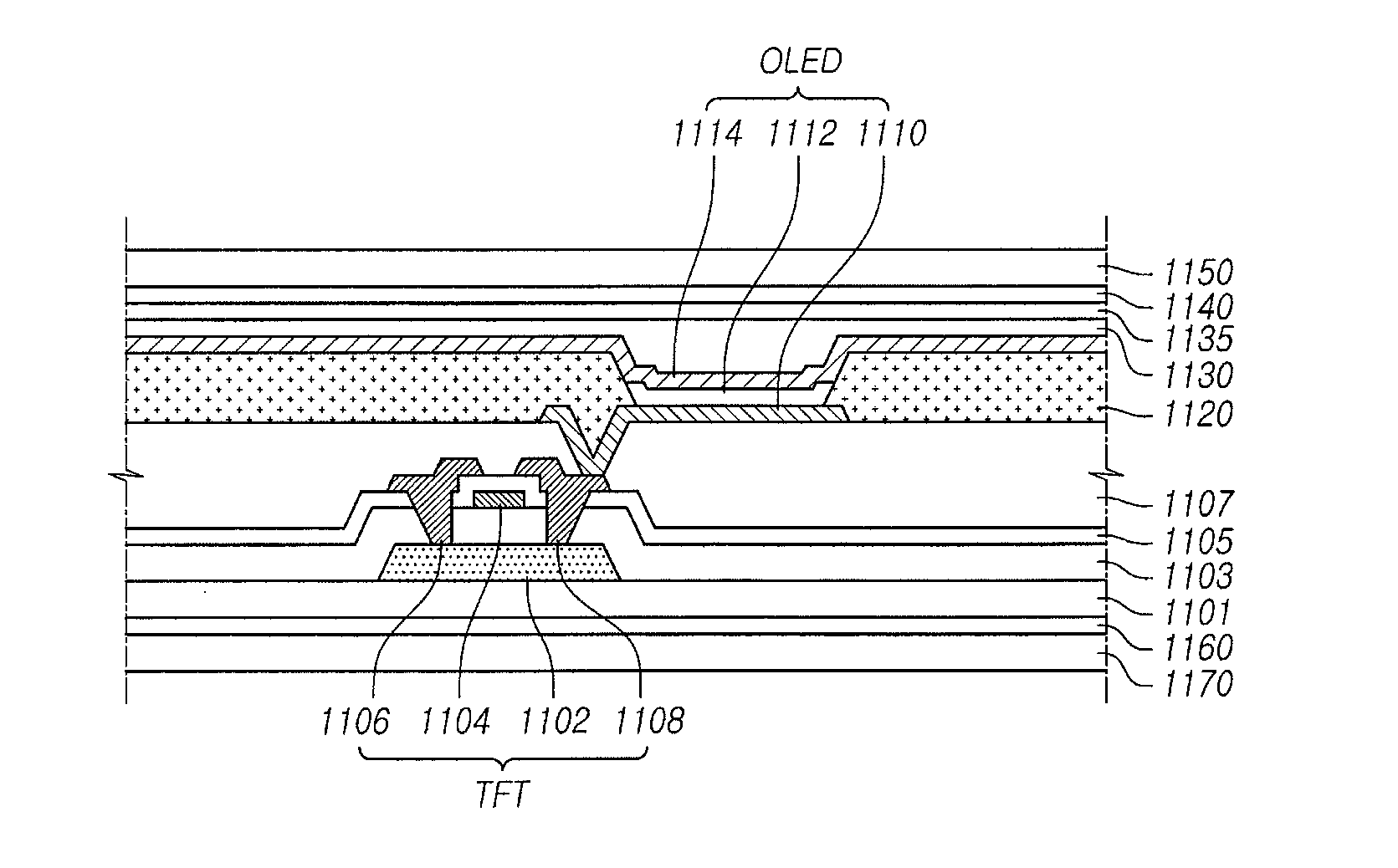
Organic light emitting display and method of fabricating the same
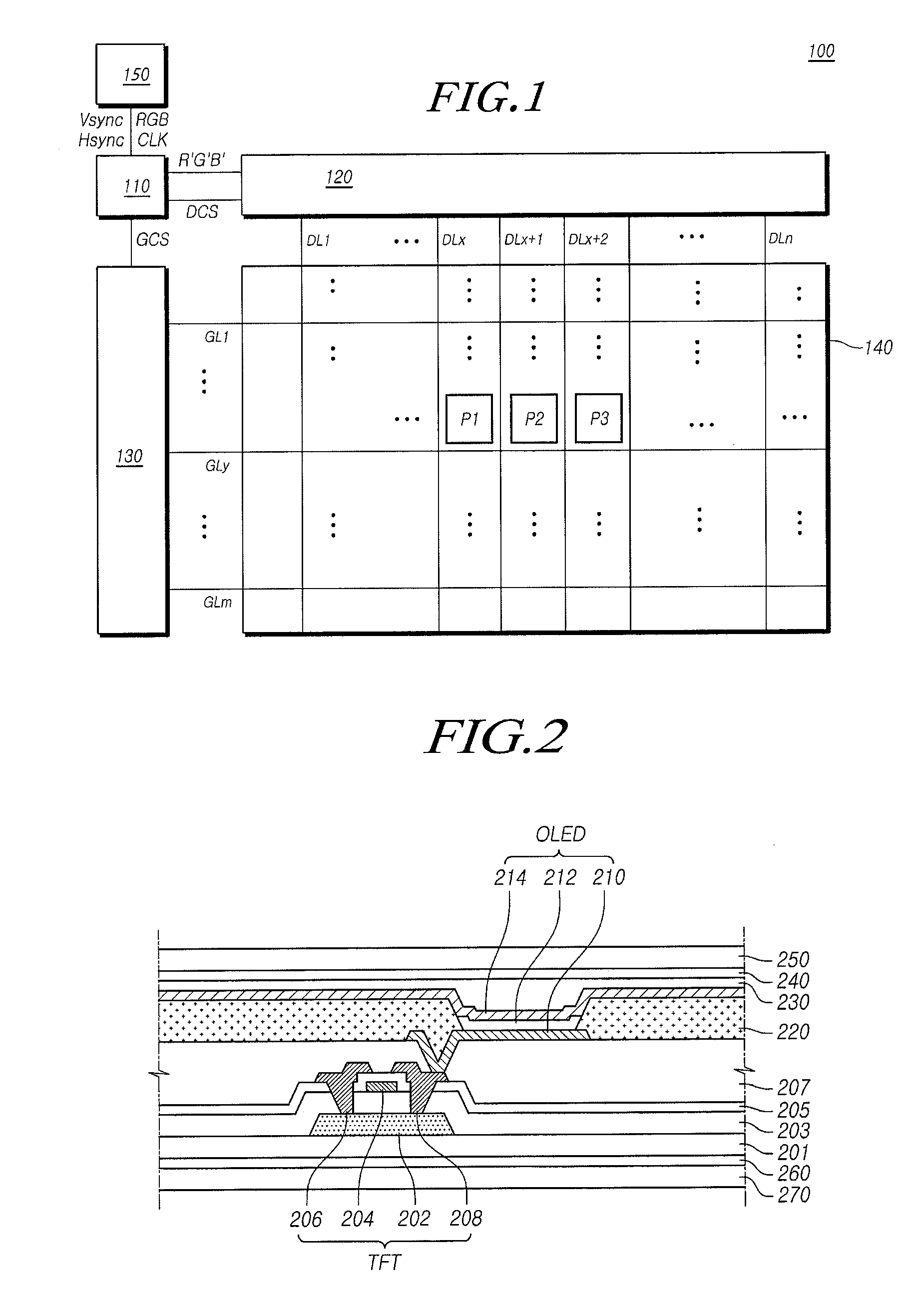
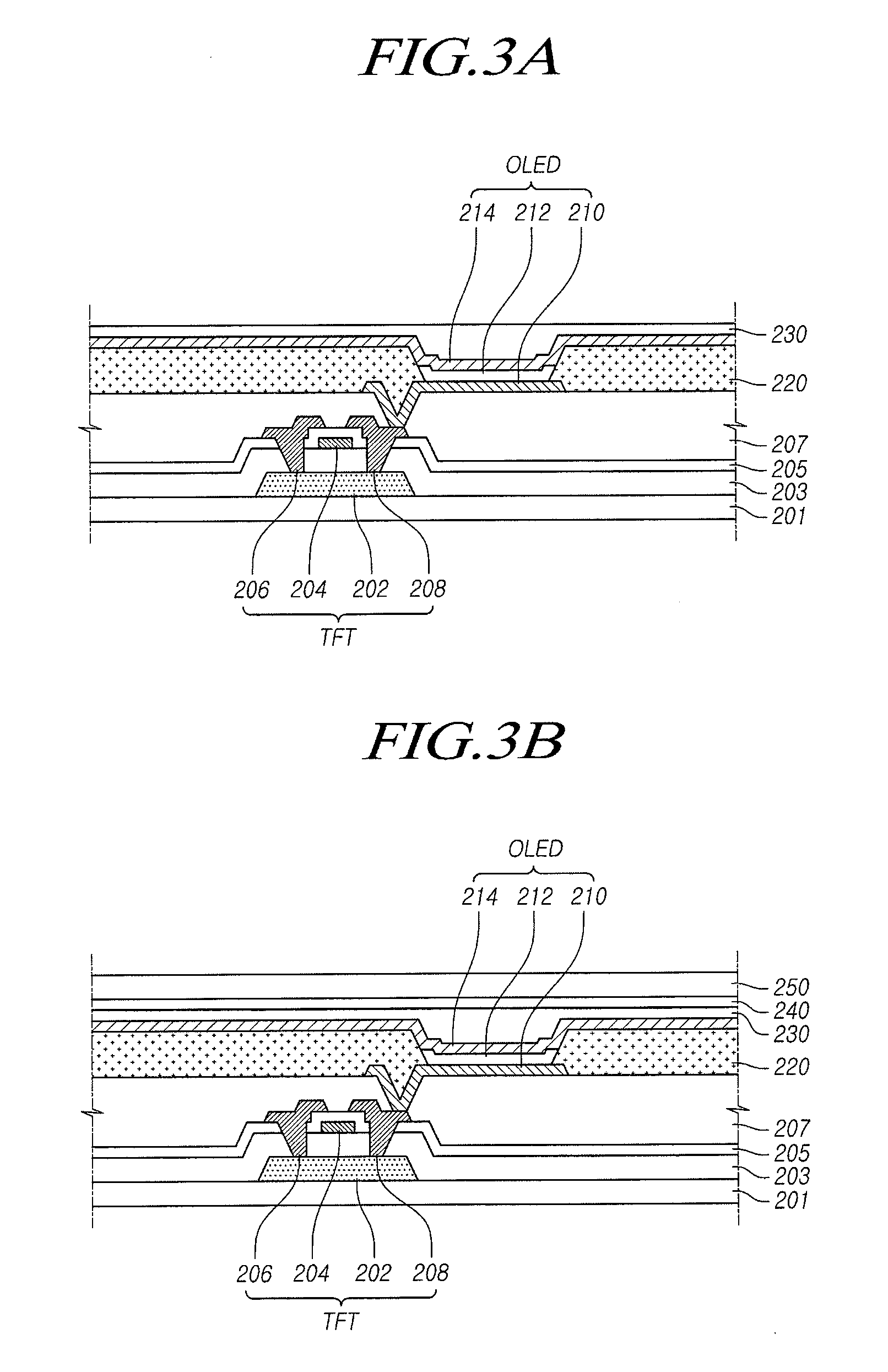
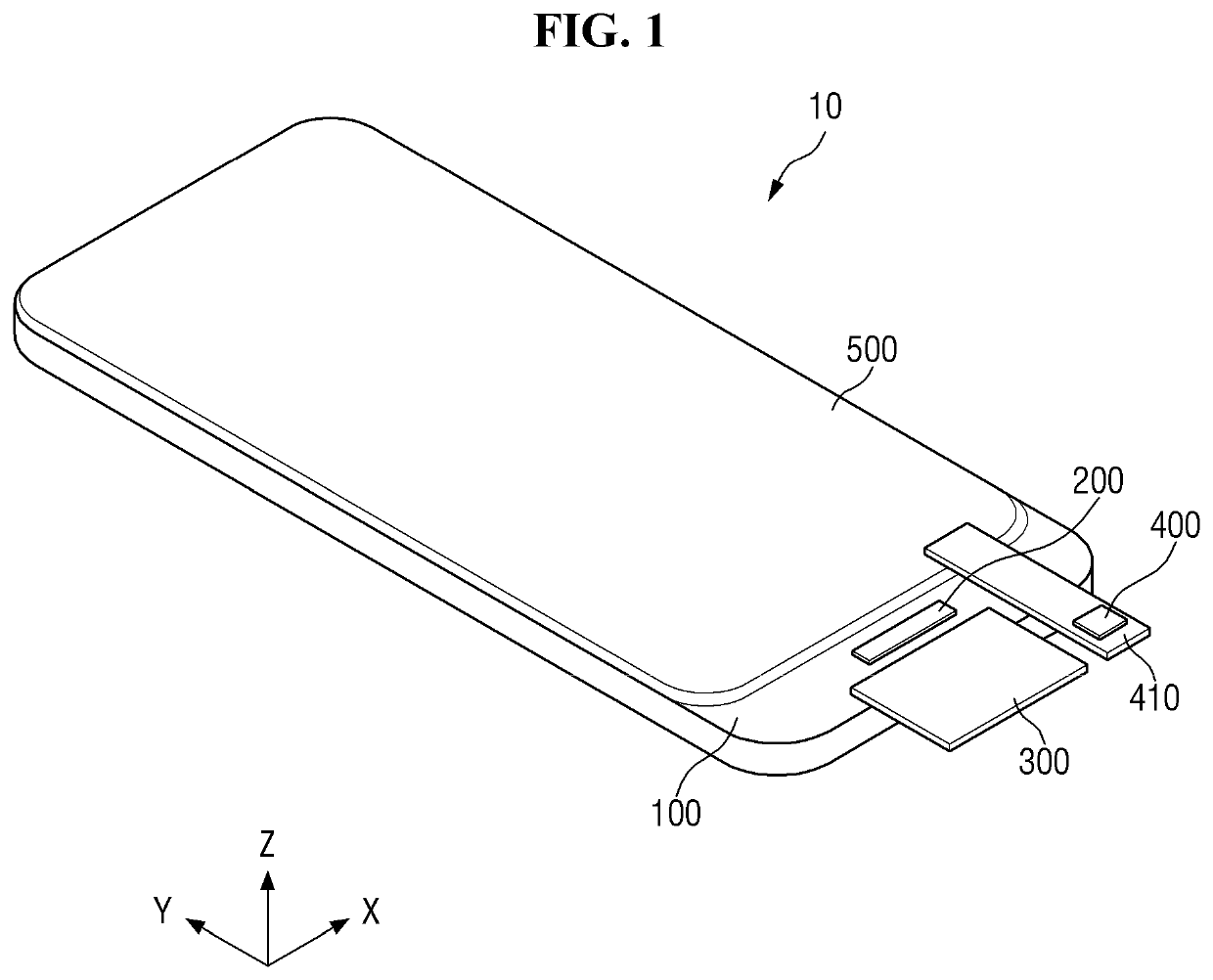
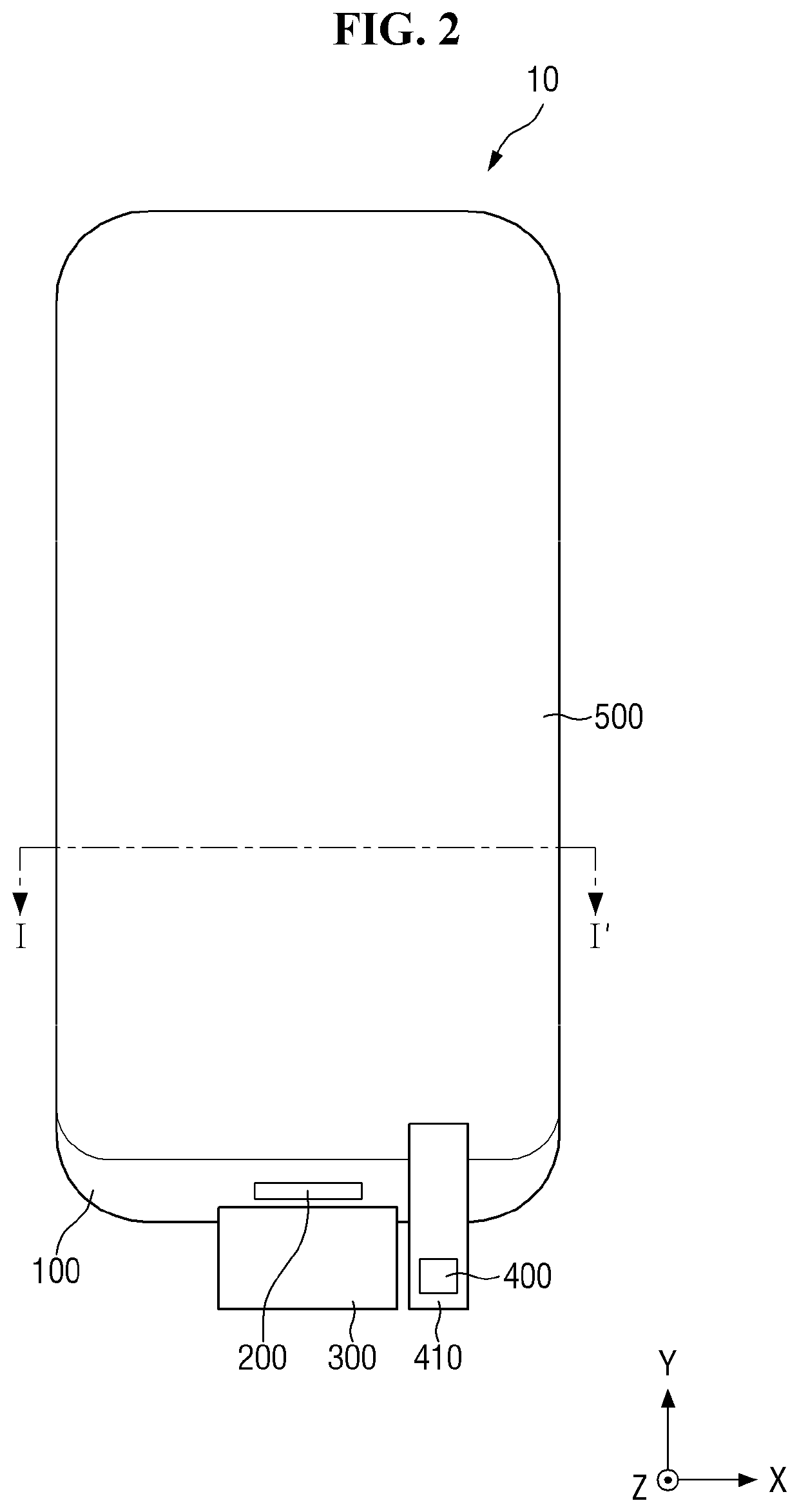
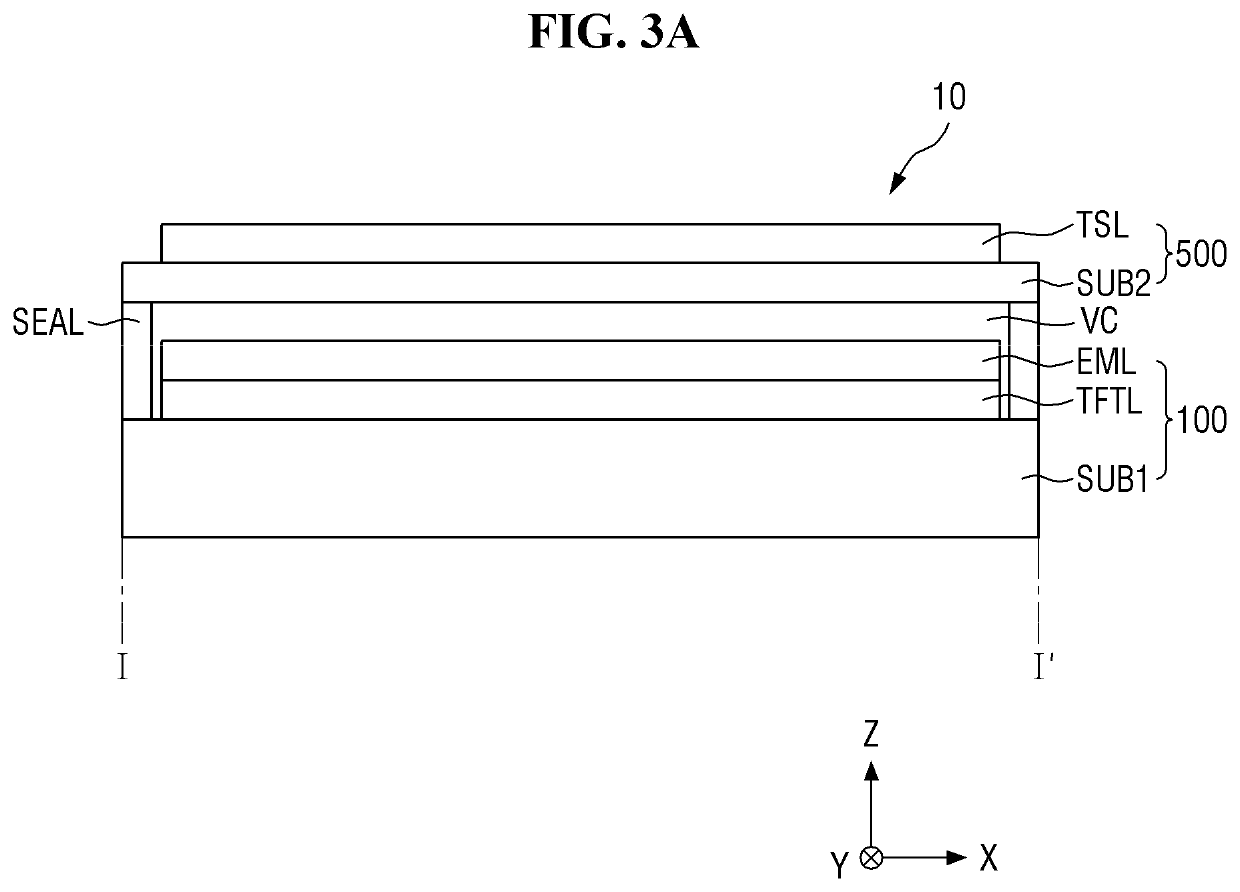
ActiveUS20150115235A1Minimize upper layerReduce and prevent occurrenceElectroluminescent light sourcesSolid-state devicesWater vaporOrganic light emitting device
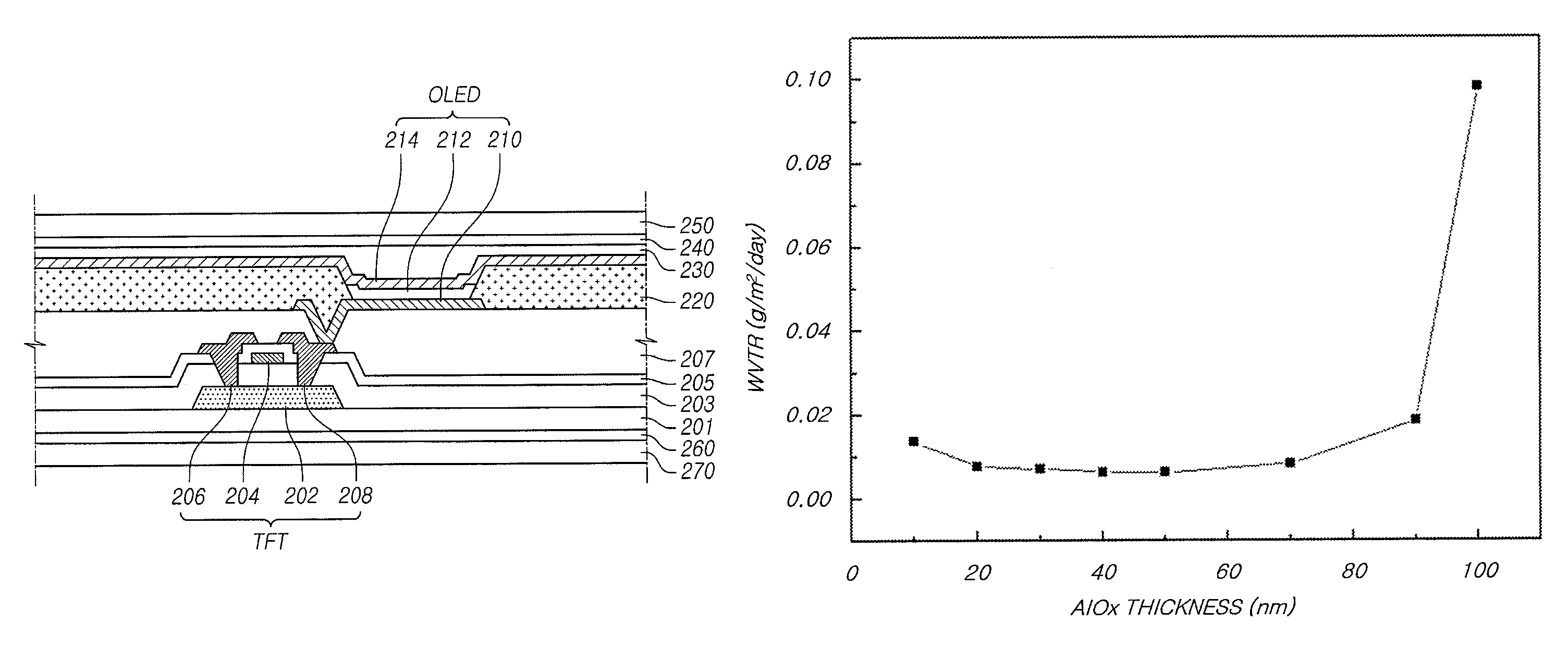
The present disclosure provides an organic light emitting display that may comprise: an organic light emitting device (OLED) including a first electrode, an organic layer including a light-emitting layer, and a second electrode, which are sequentially formed on a substrate having a Thin Film Transistor (TFT) formed on the substrate; and an upper encapsulation layer, which is formed of an aluminum oxide-based material, is formed in a single layer, and is disposed on the substrate on which the organic light emitting device (OLED) is formed, wherein a Water Vapor Transmission Rate (WVTR) of the upper encapsulation layer is smaller than or equal to 10−2 g / m2 / day.
Owner:LG DISPLAY CO LTD
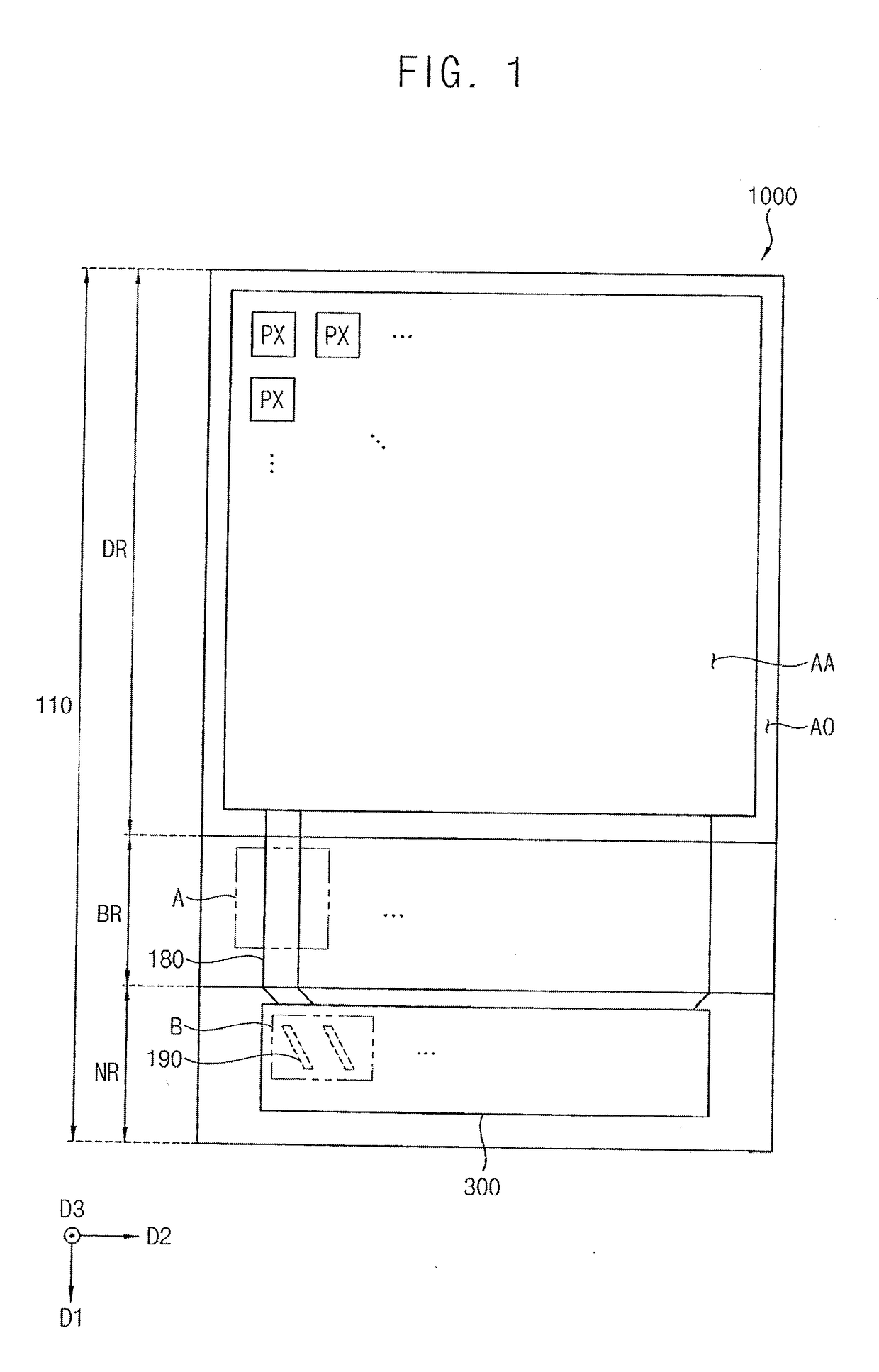
Display device having notched connection wiring
ActiveUS20180247992A1Reduce and prevent occurrenceImprove manufacturing yieldStatic indicating devicesFinal product manufactureDisplay deviceEngineering
A display device includes a substrate including a display region, a pad region spaced apart from the display region, and a bending region between the display region and the pad region. A plurality of pixel structures is positioned in the display region of the substrate. A plurality of pad wirings is positioned in the pad region of the substrate. A plurality of connection wirings electrically connect the pad wirings to the pixel structures. The connection wirings include a plurality of notches in the bending region.
Owner:SAMSUNG DISPLAY CO LTD
Ceramic multilayer substrate and manufacturing method therefor
ActiveUS20130330509A1High peel strengthImprove reliabilityCeramic layered productsPrinted circuit manufactureHigh densityMetallurgy
A ceramic multilayer substrate includes a ceramic substrate including a plurality of ceramic layers and electrodes (surface electrodes and internal electrodes) disposed on or in the ceramic layers, which are stacked on each other. A recessed portion is defined on a principal surface of any of the ceramic layers by the electrode and the surrounding ceramic layer. The electrodes (surface electrodes and internal electrodes) are buried or embedded in the ceramic layers. A peripheral portion of the surface electrode is preferably covered with a covering ceramic layer so as to prevent short-circuiting between adjacent electrodes even if surface electrodes and internal electrodes are disposed at narrow intervals and at high density.
Owner:MURATA MFG CO LTD
Detecting genetic predisposition to sight-threatening diabetic retinopathy
InactiveUS20050032077A1Reduce and prevent occurrencePredict riskMicrobiological testing/measurementGenetics predispositionGenomic DNA
A method and kit for predicting increased risk of sight-threatening diabetic retinopathy which includes isolating genomic DNA from a sample from a diabetic patient. The genetic polymorphism pattern for the genes IL-1A, IL-1B and IL-1RN is then identified in the DNA. The identified pattern is compared with control patterns of known polymorphisms, and patients expressing a genetic polymorphism pattern associated with increased risk of sight-threatening diabetic retinopathy are identified.
Owner:DUFF GORDON W +2
Articles of poly(butylene succinate) and copolymers thereof
ActiveUS20200390933A1Reduce usageInhibition of colonizationSuture equipmentsInternal osteosythesisPolymer scienceActive agent
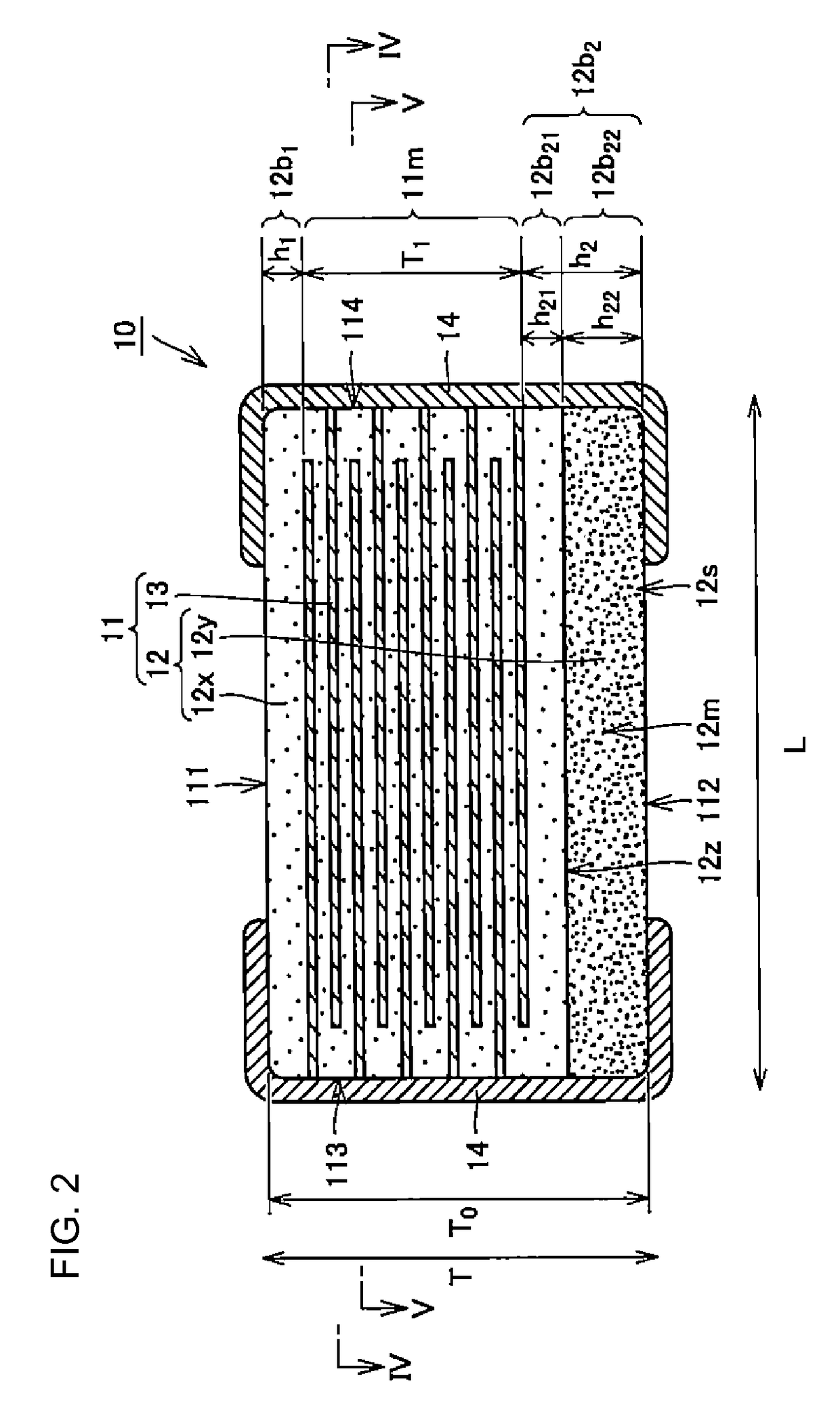
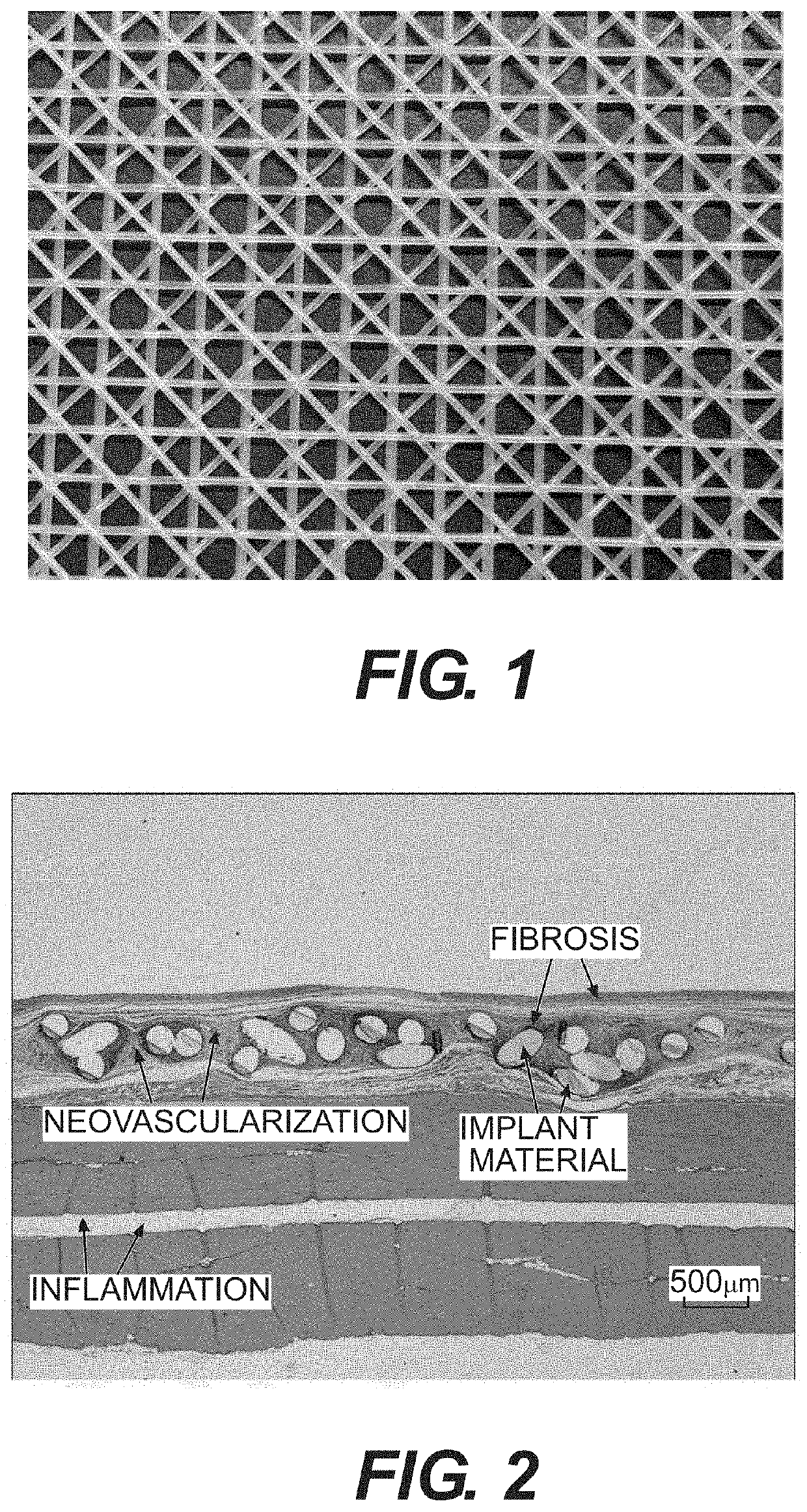
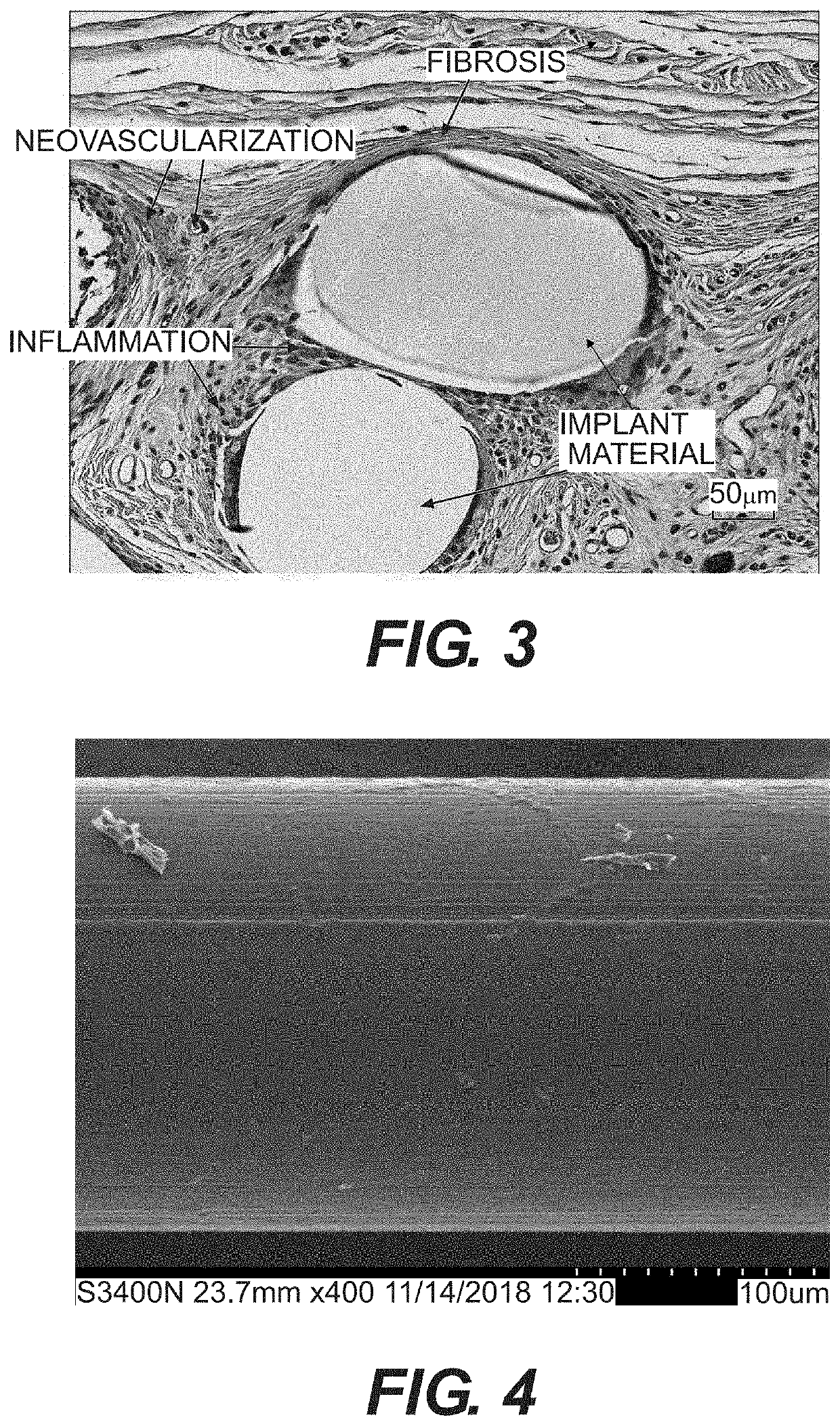
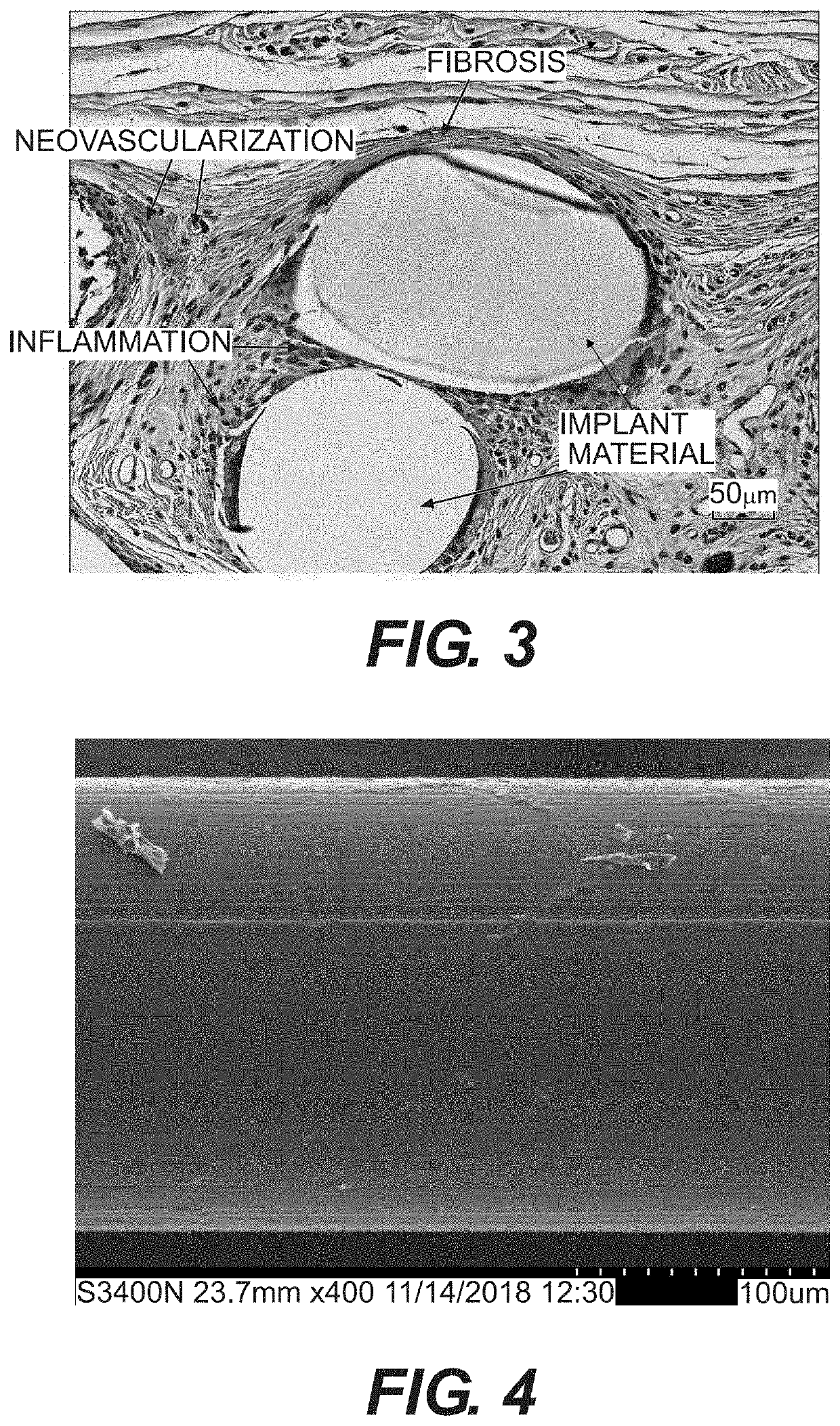
Resorbable implants, coverings and receptacles comprising poly(butylene succinate) and copolymers thereof have been developed. The implants are preferably sterilized, and contain less than 20 endotoxin units per device as determined by the limulus amebocyte lysate (LAL) assay, and are particularly suitable for use in procedures where prolonged strength retention is necessary, and can include one or more bioactive agents. The implants may be made from fibers and meshes of poly(butylene succinate) and copolymers thereof, or by 3d printing molding, pultrusion or other melt or solvent processing method. The implants, or the fibers preset therein, may be oriented. These coverings and receptacles may be used to hold, or partially / fully cover, devices such as pacemakers and neurostimulators. The coverings, receptacles and implants described herein, may be made from meshes, webs, lattices, non-wovens, films, fibers, foams, molded, pultruded, machined and 3D printed forms.
Owner:TEPHA INC
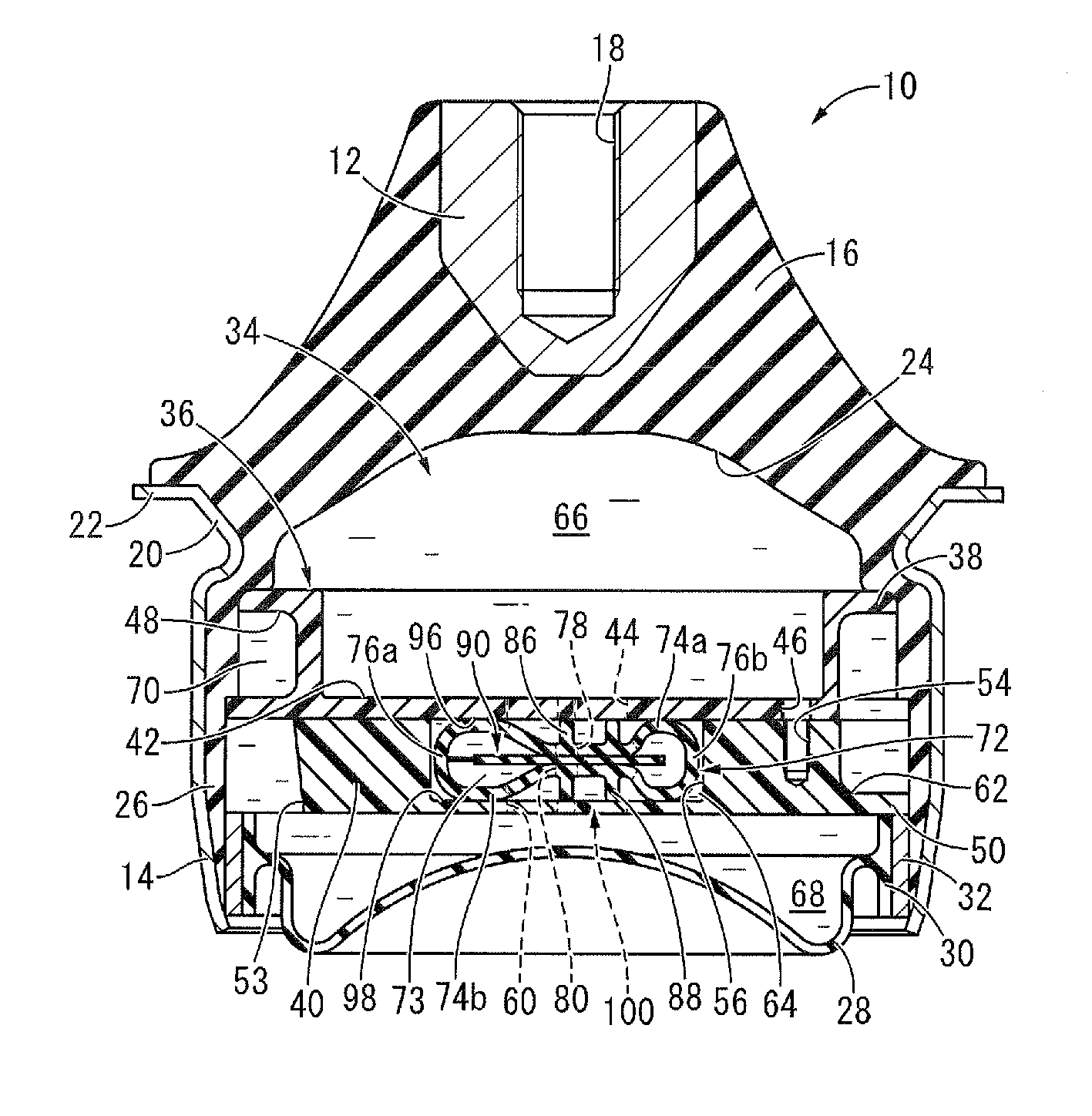
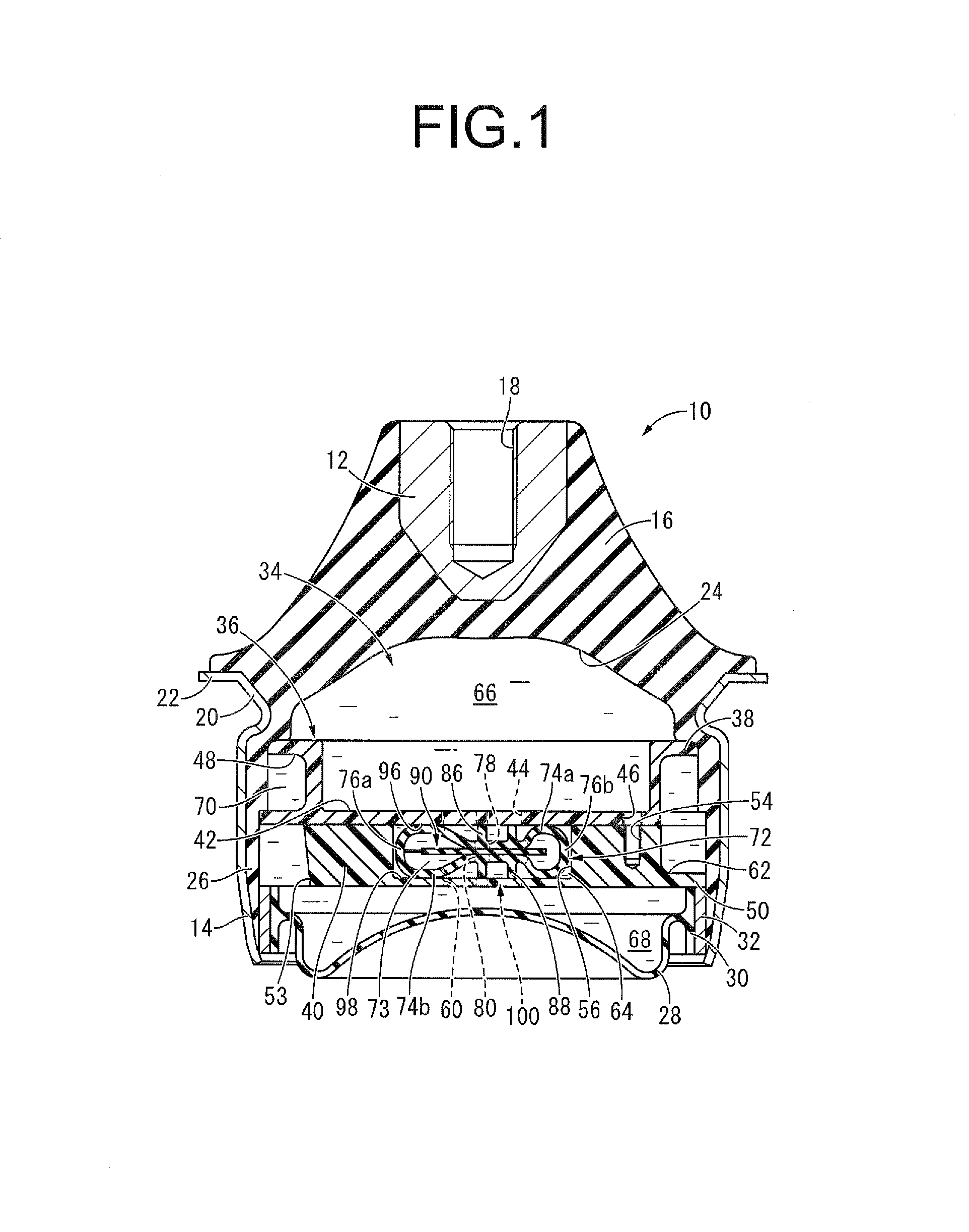
Electric power steering device
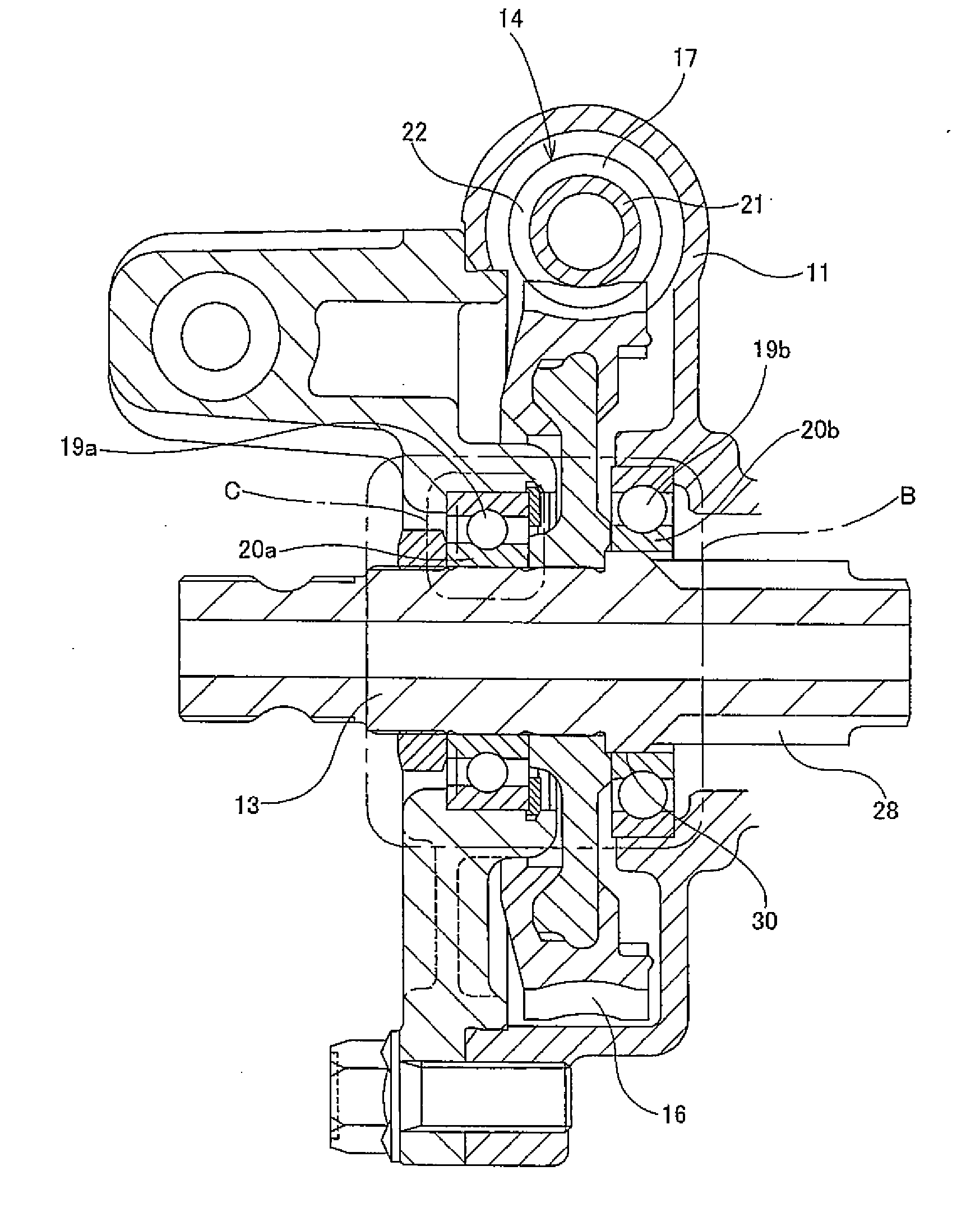
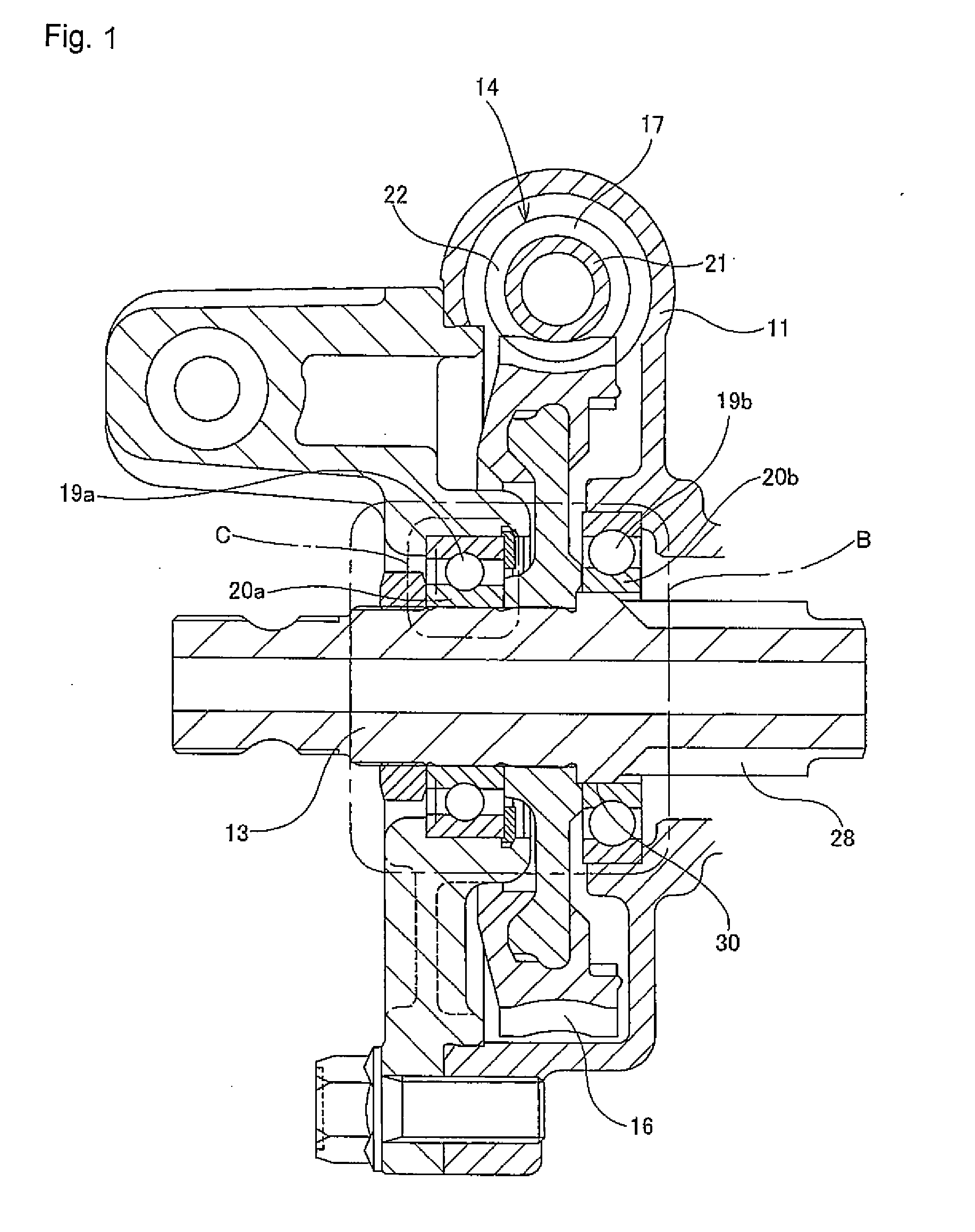
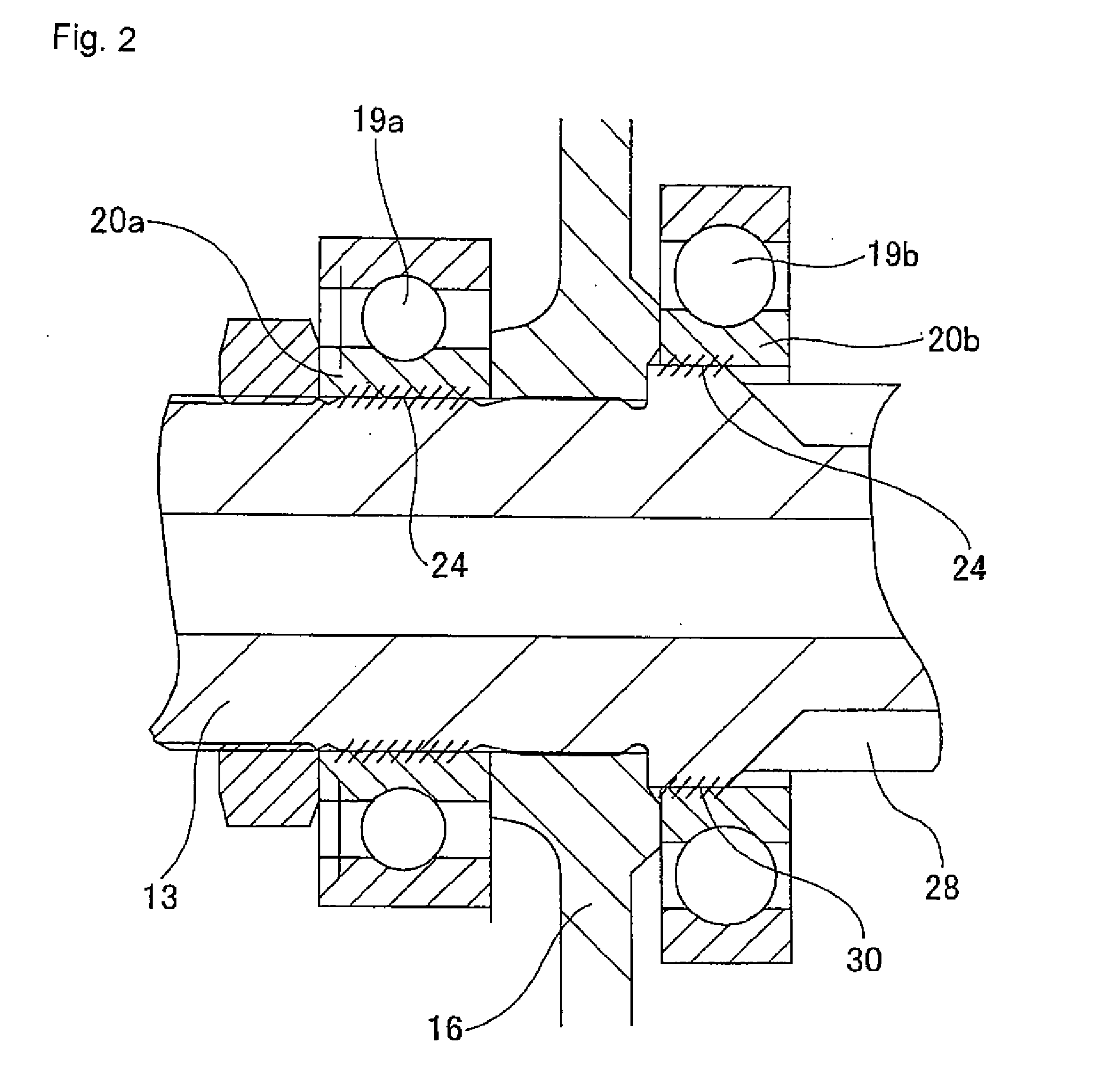
InactiveUS20110284312A1Reduction and elimination of vibrationReduction and elimination of and abnormal noiseBall bearingsSteering columnsElastomerElectric power steering
An electric power steering device is provided with a construction that can suppress the generation of stick slip between the outer peripheral face of a rotation shaft (13), and the inner peripheral face of inner rings (20a, 20b) of rolling bearings (19a, 19b). Therefore, in the present invention a lubricant (24) or an elastomer (25) is interposed between the inner peripheral face of at least one of the inner rings of the inner rings (20a, 20b), and the outer peripheral face of the rotation shaft (13). Additionally in the case where a lubricant (24) is interposed at a part of the rotation shaft (13), there is provided a surface for retaining the lubricant in this part.
Owner:NSK LTD
Tape printing apparatus with tape cassette guide members
ActiveUS20040190971A1Reduce and prevent occurrenceEasy to installInking apparatusTypewritersMagnetic tapeEngineering
Owner:BROTHER KOGYO KK
Doped ceramic materials and methods of forming the same
InactiveUS20060138715A1Reduce the amount requiredLowering of sinterabilityGlass making apparatusWorkpiecesDopantMetallurgy
A doped ceramic material comprising: a first layer comprising ceramic material and an amount of dopant, a second layer comprising the ceramic material, and a transitional layer connecting the first layer and the second layer. The transitional layer comprises the dopant in an amount which decreases in a direction from the first layer to the second layer. A method of forming the doped ceramic material is also disclosed.
Owner:NAT UNIV OF SINGAPORE
Multilayer ceramic capacitor
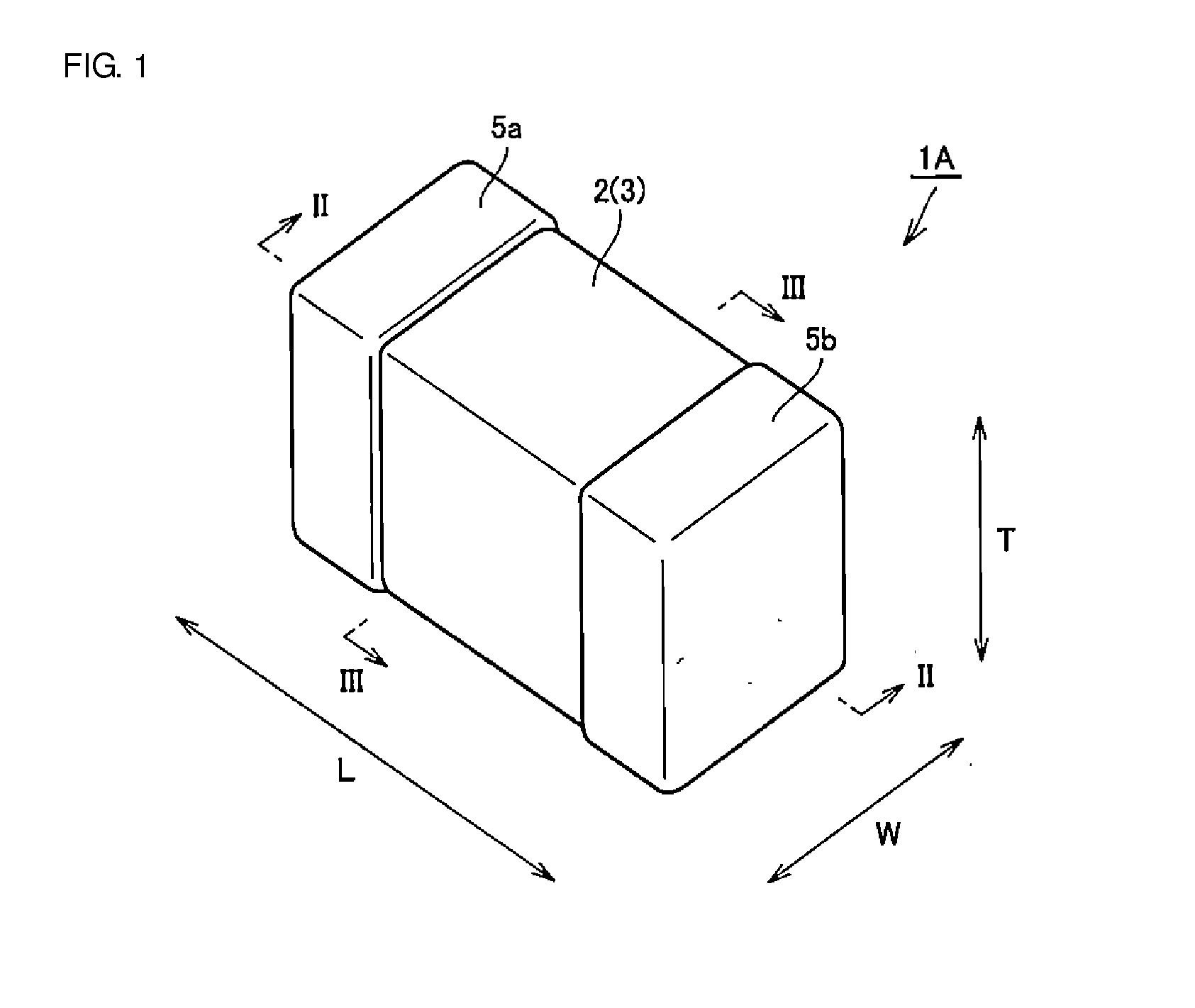
ActiveUS9627137B2Reduce and prevent occurrenceMultiple fixed capacitorsFixed capacitor dielectricCeramic capacitorDielectric layer
Owner:MURATA MFG CO LTD
Display device
ActiveUS20210303104A1Reduce and prevent likelihoodReduce and prevent occurrenceInput/output processes for data processingComputer hardwareComputer graphics (images)
Provided is a display device including a display unit including a display area, a transmitting portion surrounded by the display area, and a non-display area surrounding the display area, and a touch sensing unit having a transmissive area, dummies overlapping the transmitting portion and surrounding the transmissive area, and a touch sensor area surrounding the dummies and including first touch electrodes, and second touch electrodes respectively between the first touch electrodes, connected in a first direction, and spaced apart in a second direction perpendicular to the first direction, wherein the dummies include a main dummy surrounding the transmissive area, and at least one sub-dummy surrounding the main dummy, and wherein an outermost sub-dummy of the at least one sub-dummy that is at an outermost position of the dummies includes a first cut corresponding to a gap between adjacent first and second touch electrodes among the first and second touch electrodes.
Owner:SAMSUNG DISPLAY CO LTD
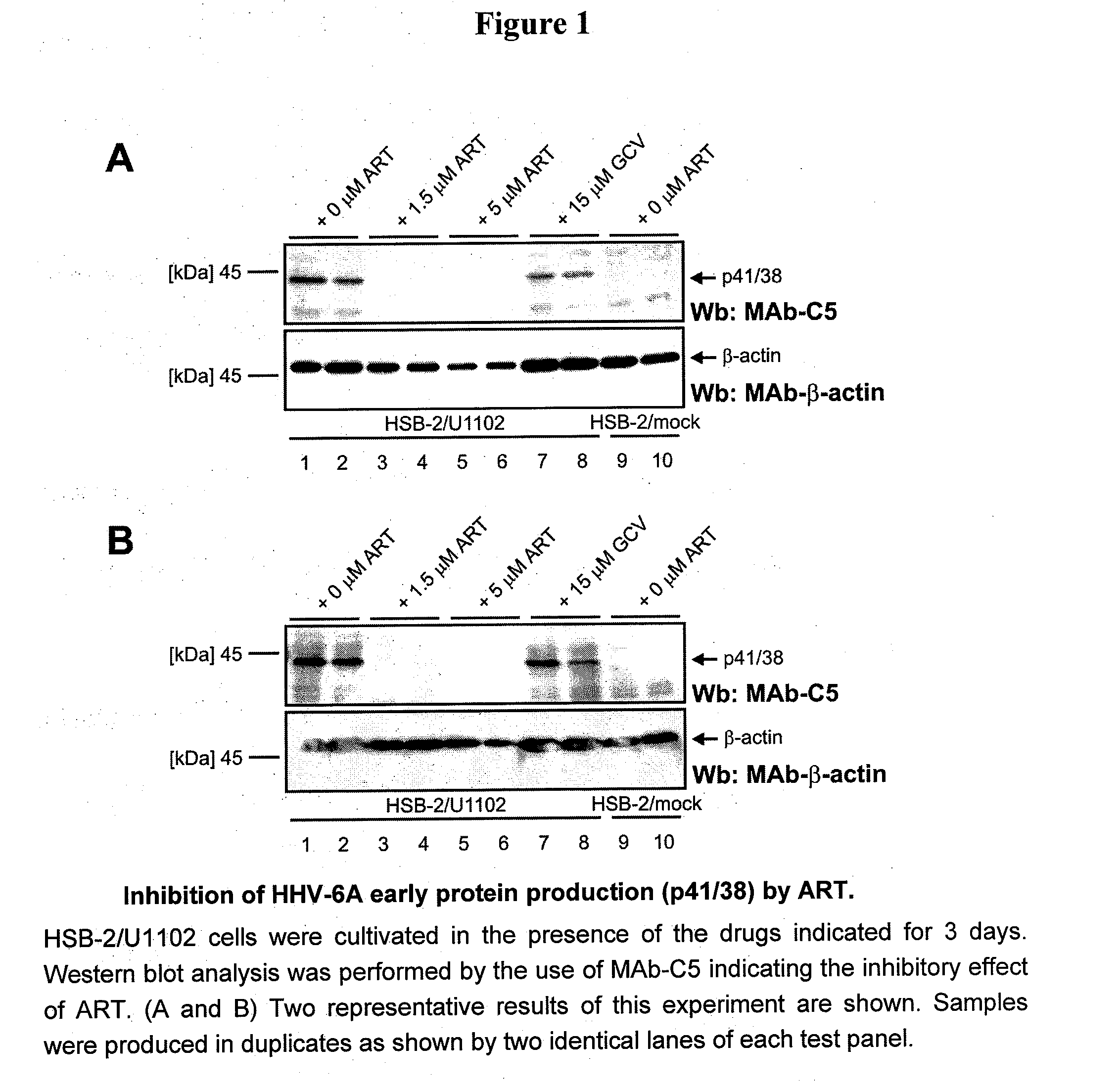
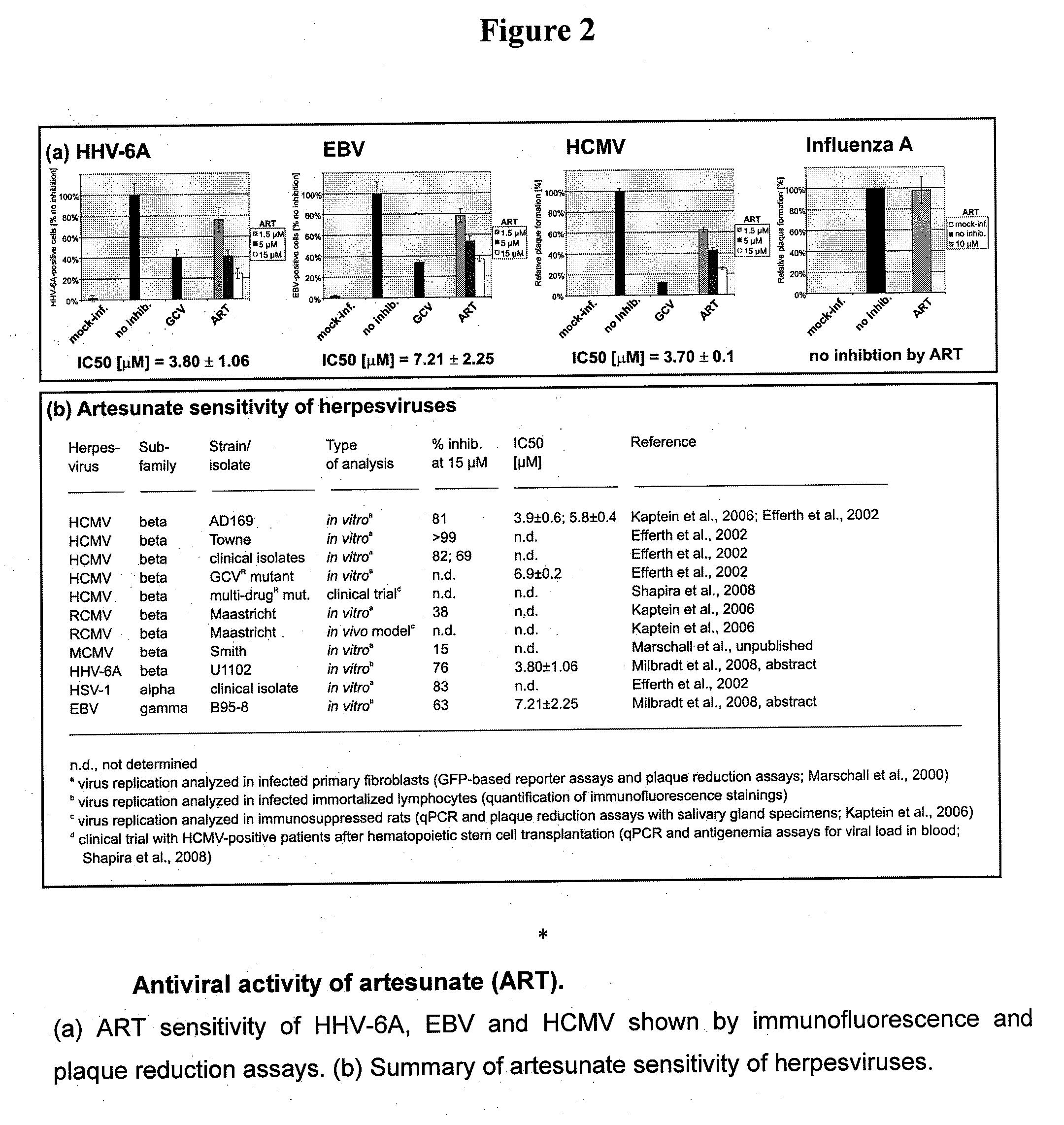
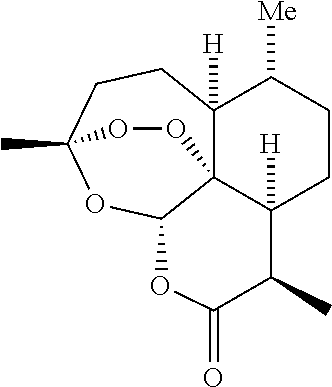
Artemisinin and derivatives thereof as antivirals
InactiveUS20110166106A1Improve securityShorten the durationBiocideOrganic chemistryNatural productMedicine
Methods to treat various herpes viral infections using the natural product artemisinin and derivatives of that compound are described. The methods are especially applicable for treatment of conditions associated with HHV-6, and are also applicable to the treatment of conditions that are induced or exacerbated by an HHV-6 infection or by a reactivation of a latent stage of an HHV-6 infection.
Owner:MARSCHALL MANFRED +2
Multilayer ceramic capacitor
ActiveUS9640323B2Reducing and preventing crackReduce and prevent occurrenceMultiple fixed capacitorsFixed capacitor dielectricCeramic capacitorEngineering
Owner:MURATA MFG CO LTD
Medical devices containing poly(butylene succinate) and copolymers thereof
PendingUS20210047484A1Reduce usageInhibition of colonizationSuture equipmentsInternal osteosythesisActive agentNonwoven fabric
Resorbable implants, coverings and receptacles comprising poly(butylene succinate) and copolymers thereof have been developed. The implants are preferably sterilized, and contain less than 20 endotoxin units per device as determined by the limulus amebocyte lysate (LAL) assay, and are particularly suitable for use in procedures where prolonged strength retention is necessary, and can include one or more bioactive agents. The implants may be made from fibers and meshes of poly(butylene succinate) and copolymers thereof, or by 3d printing molding, pultrusion or other melt or solvent processing method. The implants, or the fibers preset therein, may be oriented. These coverings and receptacles may be used to hold, or partially / fully cover, devices such as pacemakers and neurostimulators. The coverings, receptacles and implants described herein, may be made from meshes, webs, lattices, non-wovens, films, fibers, foams, molded, pultruded, machined and 3D printed forms.
Owner:TEPHA INC
Organic light emitting display and method of fabricating the same
ActiveUS9461269B2Minimize upper layerReduce and prevent occurrenceElectroluminescent light sourcesSolid-state devicesWater vaporOrganic light emitting device
The present disclosure provides an organic light emitting display that may comprise: an organic light emitting device (OLED) including a first electrode, an organic layer including a light-emitting layer, and a second electrode, which are sequentially formed on a substrate having a Thin Film Transistor (TFT) formed on the substrate; and an upper encapsulation layer, which is formed of an aluminum oxide-based material, is formed in a single layer, and is disposed on the substrate on which the organic light emitting device (OLED) is formed, wherein a Water Vapor Transmission Rate (WVTR) of the upper encapsulation layer is smaller than or equal to 10−2 g / m2 / day.
Owner:LG DISPLAY CO LTD
Medical devices containing compositions of poly(butylene succinate) and copolymers thereof
PendingUS20210046212A1Reduce usageInhibition of colonizationSuture equipmentsInternal osteosythesisActive agentNonwoven fabric
Resorbable implants, coverings and receptacles comprising poly(butylene succinate) and copolymers thereof have been developed. The implants are preferably sterilized, and contain less than 20 endotoxin units per device as determined by the limulus amebocyte lysate (LAL) assay, and are particularly suitable for use in procedures where prolonged strength retention is necessary, and can include one or more bioactive agents. The implants may be made from fibers and meshes of poly(butylene succinate) and copolymers thereof, or by 3d printing molding, pultrusion or other melt or solvent processing method. The implants, or the fibers preset therein, may be oriented. These coverings and receptacles may be used to hold, or partially / fully cover, devices such as pacemakers and neurostimulators. The coverings, receptacles and implants described herein, may be made from meshes, webs, lattices, non-wovens, films, fibers, foams, molded, pultruded, machined and 3D printed forms.
Owner:TEPHA INC
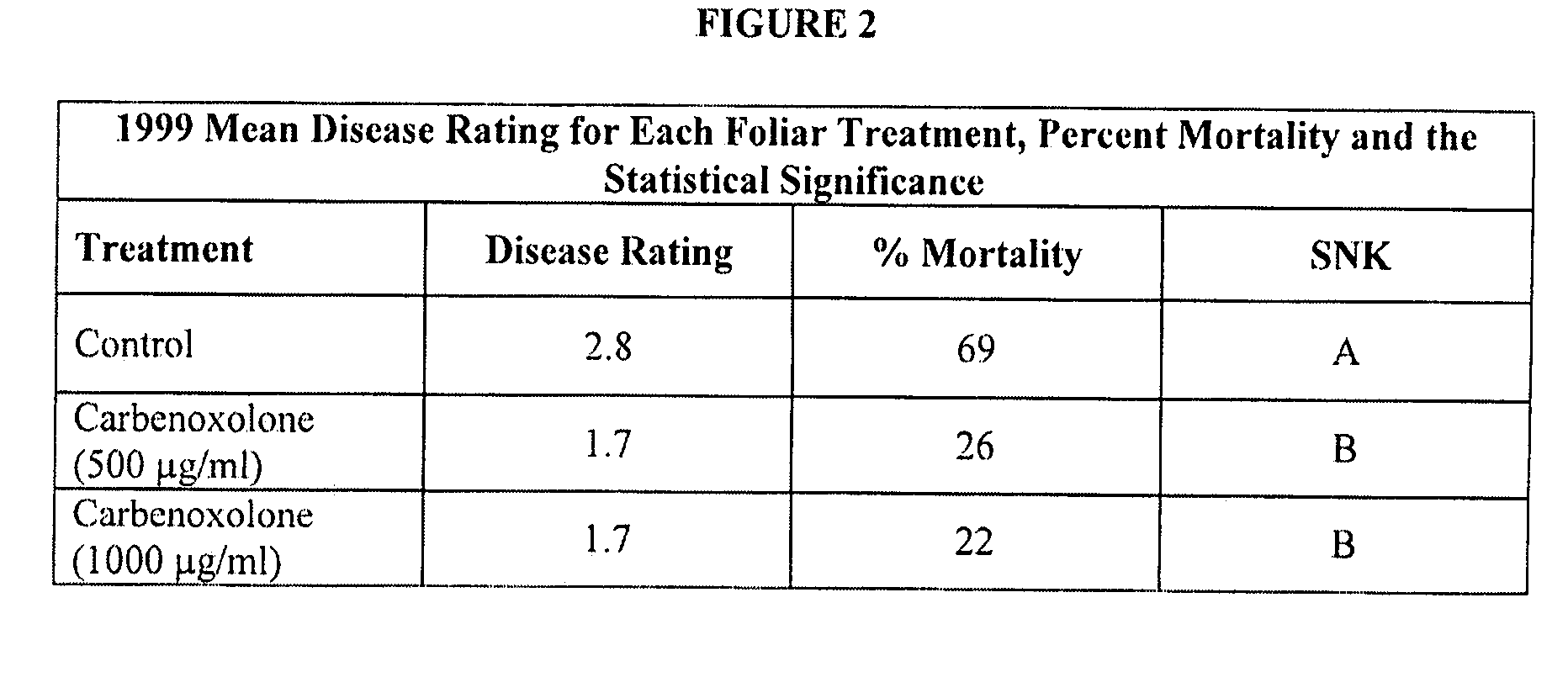
Methods and Compositions for the Management of Soil Borne Fungal Diseases
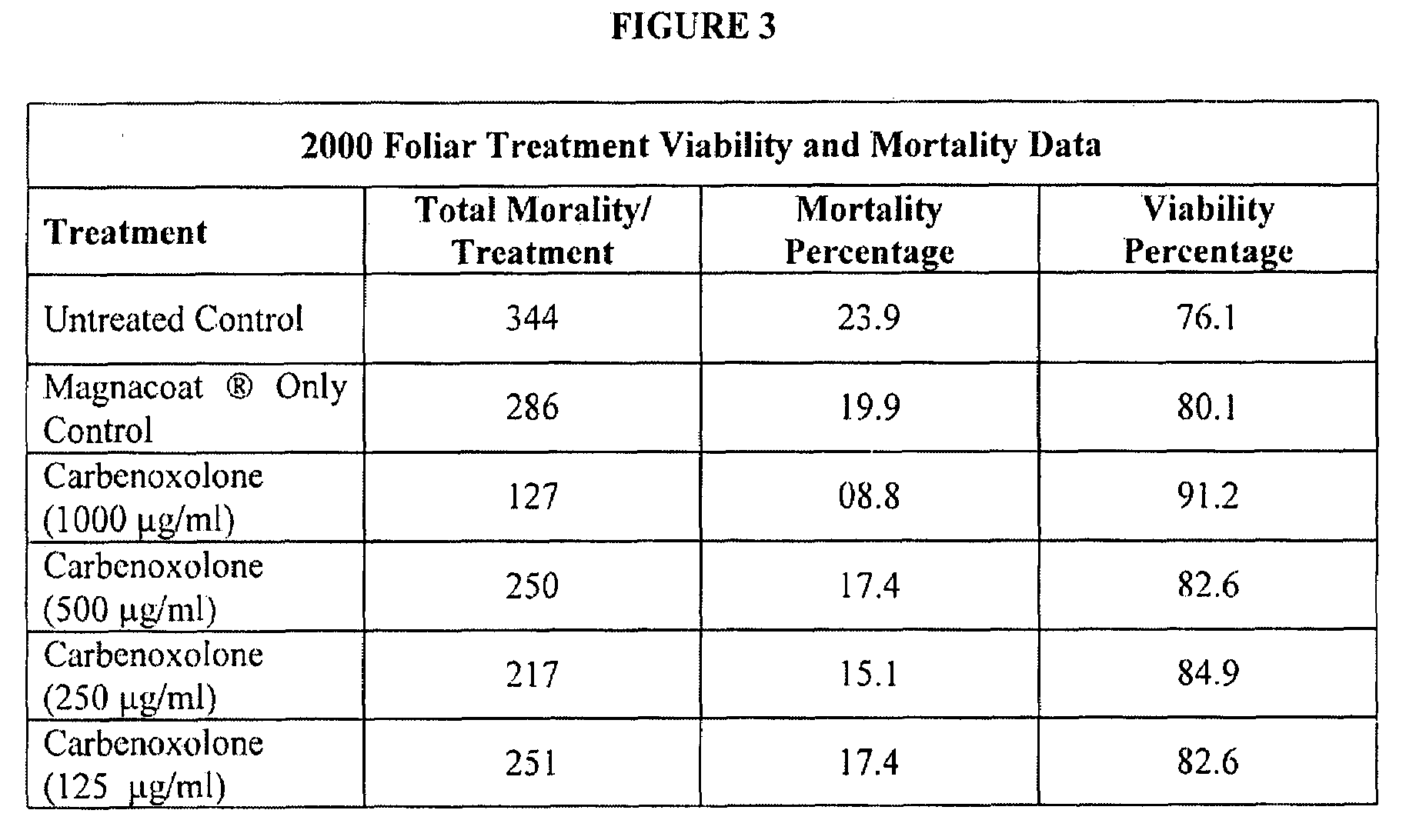
InactiveUS20090099262A1Lower Level RequirementsReduce and prevent occurrenceBiocideDead animal preservationParticulatesWater insoluble
Improved methods and compositions for protecting plants or seeds from soil-borne fungal diseases. The composition may include a triterpenoid isolated from Glycyrrhiza glabra and a polymer. The triterpenoid may be Carbenoxolone disodium salt, and the polymer may be a water-insoluble, water-soluble or flowable, seed coating polymer. The methods may comprise the steps of applying the composition to the plant's seeds, roots, tubers and / or foliage, and may also include applying the composition to the soil surround surrounding the plant. The composition may be applied as an aqueous solution or as dry particulates, and may be used for the treatment of soybean plants and seeds.
Owner:PITTSBURG STATE UNIVERSITY
Multilayer ceramic capacitor
ActiveUS9659712B2Reduce and prevent occurrenceFixed capacitor electrodesFixed capacitor dielectricCeramic capacitorBoundary region
A multilayer ceramic capacitor includes a body and at least two outer electrodes. The body includes first and second main surfaces, an inner layer portion and first and second outer layer portions. In the inner layer portion, dielectric layers and conductive layers are alternately stacked on each other. The second outer layer portion includes an outer portion and an inner portion. A boundary region adjacent to the inner portion in the outer portion inclines toward the first main surface.
Owner:MURATA MFG CO LTD
Yarns and fibers of poly(butylene succinate) and copolymers thereof, and methods of use therof
PendingUS20190269815A1Inhibition of colonizationReduce and prevent occurrenceSuture equipmentsMammary implantsYarnBiomedical engineering
Resorbable implants comprising poly(butylene succinate) and copolymers thereof have been developed. The implants implants are preferably sterilized, and contain less than 20 endotoxin units per device as determined by the limulus amebocyte lysate (LAL) assay, and are particularly suitable for use in procedures where prolonged strength retention is necessary, and can include one or more bioactive agents. The implants may be made from fibers and meshes of poly(butylene succinate) and copolymers thereof, or by 3d printing, and the fibers may be oriented. Coverings and receptacles made from forms of poly(butylene succinate) and copolymers thereof have also been developed for use with cardiac rhythm management devices and other implantable devices. These coverings and receptacles may be used to hold, or partially / fully cover, devices such as pacemakers and neurostimulators. The coverings and receptacles are made from meshes, webs, lattices, non-wovens, films, fibers, and foams, and contain antibiotics such as rifampin and minocycline.
Owner:TEPHA INC
Multilayer ceramic capacitor, multilayer ceramic capacitor series including the same, and multilayer ceramic capacitor mount body including the same
ActiveUS9728336B2Reduce and prevent occurrenceSufficient electrostatic capacitanceMultiple fixed capacitorsFixed capacitor electrodesCeramic capacitorDielectric layer
A body of a multilayer ceramic capacitor includes an inner layer portion and first and second outer layer portions sandwiching the inner layer portion therebetween. The inner layer portion includes an area extending from a conductive layer positioned closest to a first main surface to a conductive layer positioned closest to a second main surface in the stacking direction. The height of the body is smaller than the width of the body. The height of the inner layer portion is smaller than the width of the inner layer portion. The first outer layer portion includes a dielectric layer positioned closest to the first main surface. The second outer layer portion includes a dielectric layer positioned closest to the second main surface, and is thicker than the first outer layer portion. The total height of the first and second outer layer portions is smaller than the height of the inner layer portion.
Owner:MURATA MFG CO LTD
Fluid-filled vibration damping device
ActiveUS20140327199A1Reduce free lengthReduce and avoidMachine framesLiquid springsElastomerEngineering
Owner:SUMITOMO RIKO CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com