TNF (Tumor Necrosis Factor)-related apoptosis-inducing ligand fusion protein and preparation method thereof
A technology of apoptosis-inducing ligand and tumor necrosis factor, which is applied in the field of tumor necrosis factor-related apoptosis-inducing ligand fusion protein and its preparation to achieve the effects of prolonging half-life, inhibiting angiogenesis and maintaining apoptosis activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
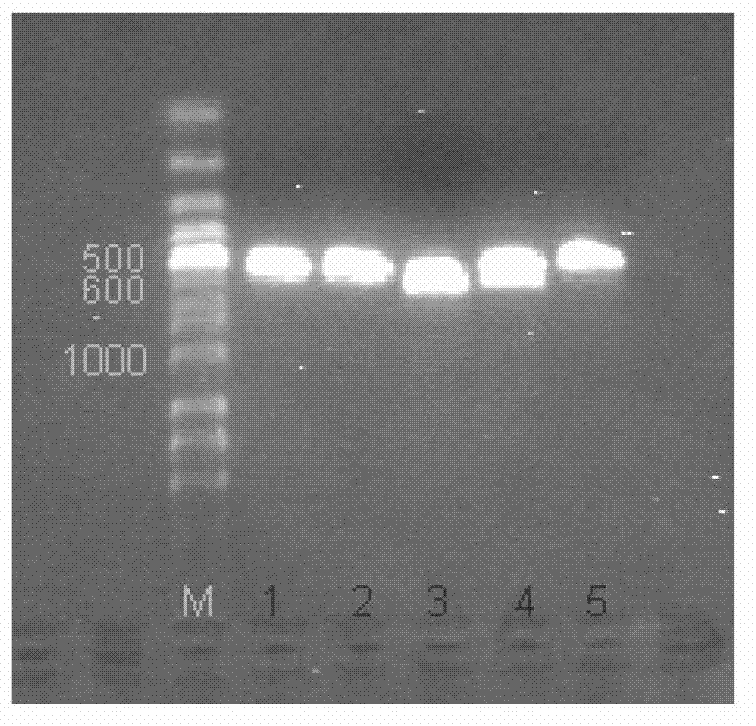
[0030] Example 1: Amplification of TRAIL114-281 and NC1 fragments
[0031] (1) Amplification of TRAIL114-281
[0032] Extract human prostate total mRNA: Grind frozen human prostate tissue small pieces (about 100mg) into powder with liquid nitrogen, add 1ml TRIZOL reagent and continue grinding repeatedly, transfer to 1.5ml RNase-free EP tube, place at room temperature for 5min, add 0.2 Vigorously shake in ml chloroform for 15 seconds, and place at room temperature for 3 minutes. Centrifuge at 12000g at 4°C for 15 minutes, and transfer the upper aqueous phase to another RNase-free EP tube. Add 0.5ml of isopropanol and let stand at room temperature for 10min to precipitate RNA. Centrifuge at 12,000 g at 4°C for 10 min to remove the supernatant, and wash the precipitate with 75% ethanol. Centrifuge at 12,000 g at 4°C for 5 minutes to remove the supernatant, dissolve in 50 μl DEPC water after drying, and store at -70°C. Using oligodT as primer, human prostate total RNA as temp...
Embodiment 2
[0035] Example 2: Construction of TRAIL-(G)n-NC1 and TRAIL-(G)n-TD fusion protein expression vectors and their prokaryotic expression
[0036] ⑴ Synthetic protein expression vector construction:
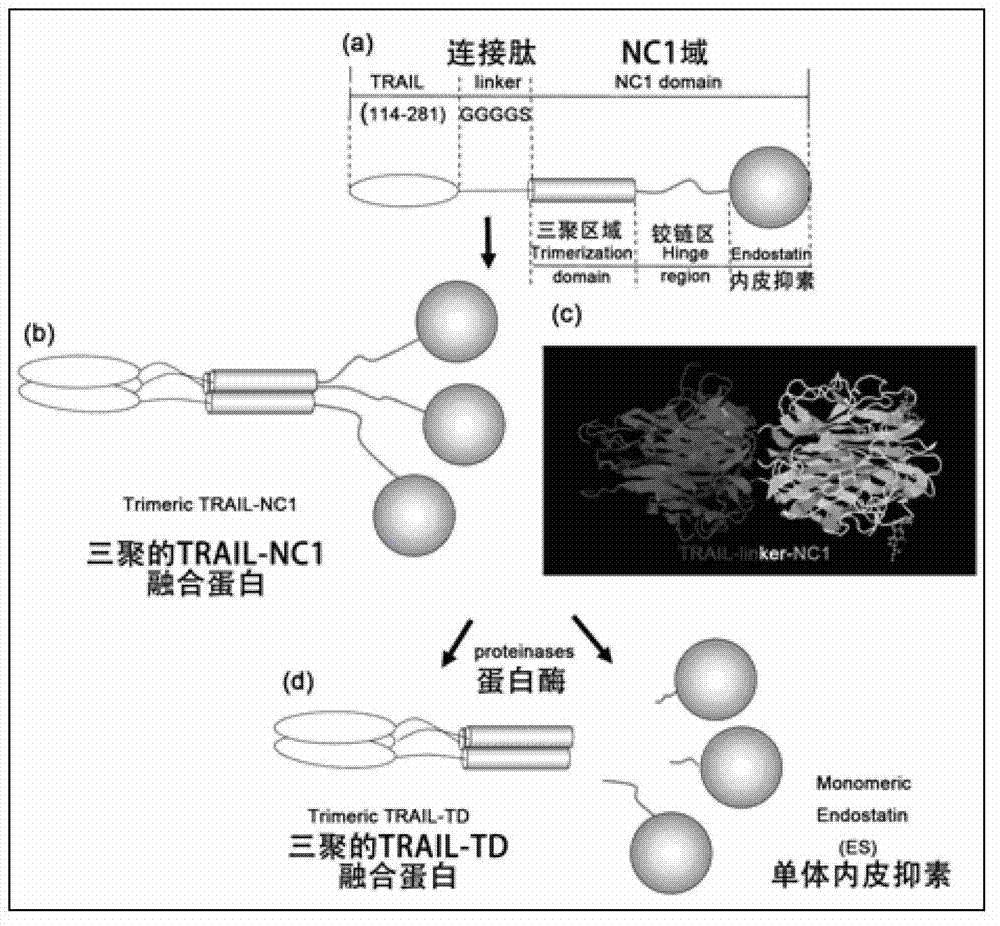
[0037] The pET28a vector contains NcoI, BamHI and XhoI, and TRAIL114-281 was digested with NcoI and BamHI and ligated with pET28a after the same digestion to construct pET28a-TRAIL114-281. The pET28a-TRAIL114-281 fragment was ligated to pET28a-TRAIL114-281 after double digestion of N1 with BamHI and XhoI to construct the pET28a-TRAIL(114-281)-linker-NC1 expression vector. see image 3 : (a): Monomer composition of TRAIL-(G)n-NC1 fusion protein: TRAIL114-281, peptide linker and NC1 domain. The NC1 domain is composed of three parts: trimerization domain (TD), hinge region and endostatin (endostatin); (b): schematic diagram of TRAIL-(G)n-NC1 fusion protein trimer; (c): TRAIL- (G) Ideal three-dimensional structure diagram of n-NC1 trimer; (d): Ideal schematic diagram of TRAIL-(G)n-...
Embodiment 3
[0040] Example 3: Acquisition and preliminary purification of TRAIL-(G)n-NC1 and TRAIL-(G)n-TD fusion protein inclusion bodies
[0041]Resuspend the cells with 50mM PBS (pH 7.4), 20mM EDTA, 0.5-3% (v / v) Triton X-100, vortex for 5min, crush BL21 mechanically with French pressure, centrifuge to remove the supernatant, and obtain crude inclusion bodies. Wash with 50mM PBS (pH 7.4), 1M NaCl, 1-6 M Urea, 0.5-3% (v / v) Triton X-100, and centrifuge. repeat three times. Wash with sterile water three times. Wash again with 50mM PBS (pH 7.4), centrifuge to remove the supernatant, and the obtained fusion protein inclusion body is initially purified. Dissolve inclusion bodies with 8-10M Urea or 5-8M GdmCl+50mM NaH2PO4+30-300 mM DTT (pH 8-10), let stand at room temperature for 5 hours, centrifuge to take the supernatant, which is the dissolved inclusion bodies.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com