Anti-tumor fusion protein, and preparation method and application thereof
A technology of fusion protein and prosthetic protein, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053]Embodiment 1, preparation recombinant expression construct
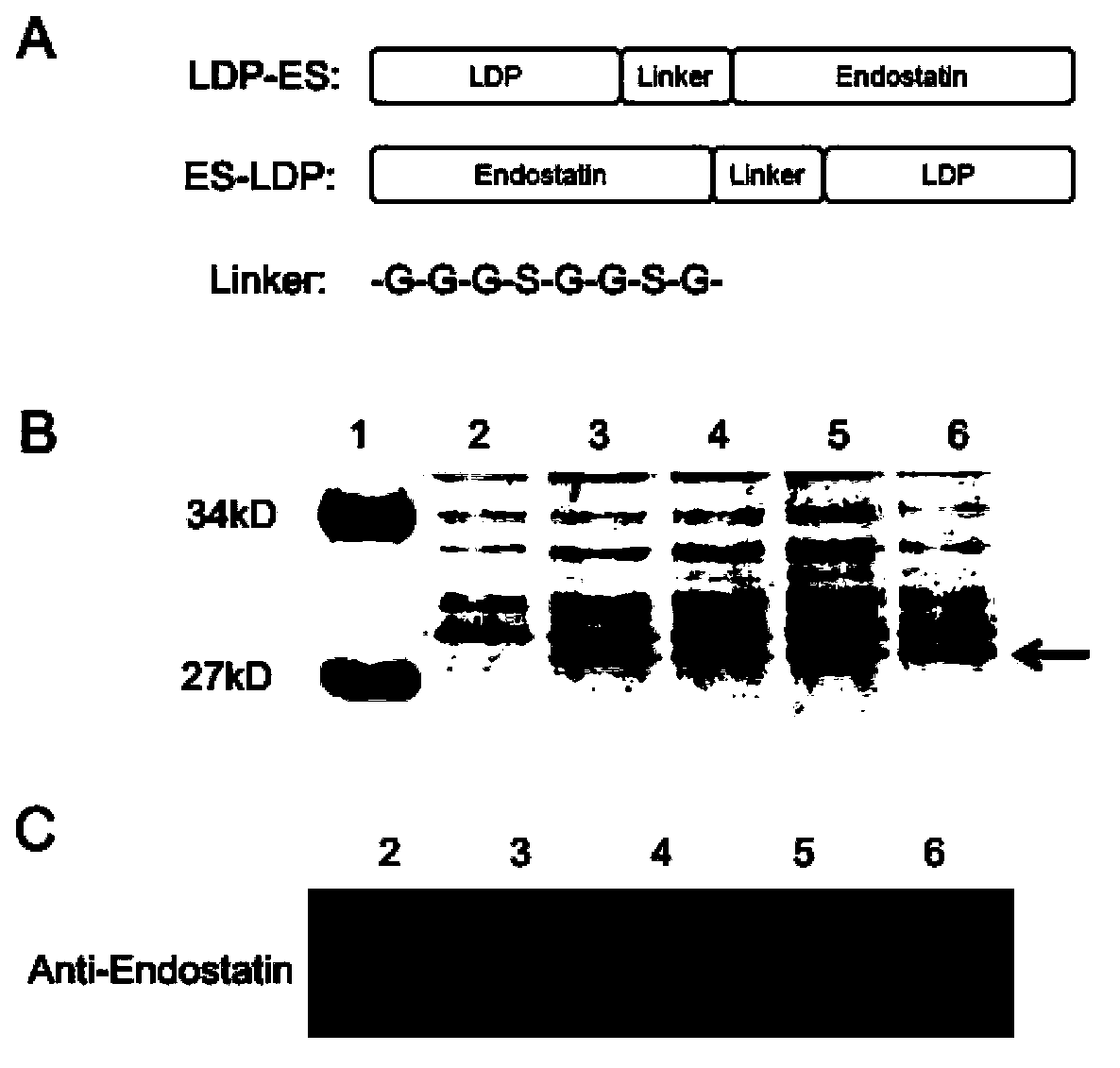
[0054] The DNA coding frame of the fusion protein composed of LDP and endostatin is prepared by PCR and molecular cloning techniques. The full-length coding sequence of fusion protein comprises the nucleotide sequence (SEQ ID NO: 8) of coding LDP and the nucleotide sequence (SEQ ID NO: 9) of coding endostatin, the sequence (SEQ ID NO: 9) by coding connecting peptide GGGSGGSG in the middle ID NO:10) separated. The obtained full-length coding sequence was inserted into the expression vector of pET30a to obtain an expression construct. like figure 1 As shown in A, two fusion proteins named ES-LDP (SEQ ID NO: 4) and LDP-ES (SEQ ID NO: 5) were constructed. The polynucleotide fragments (SEQ ID NO:6 and SEQ ID NO:7) encoding these two fusion proteins were respectively inserted into the pET30a expression vector, and then transformed into BL21(DE3) competent cells.
Embodiment 2
[0055] Example 2, expression, purification, renaturation and identification of fusion protein
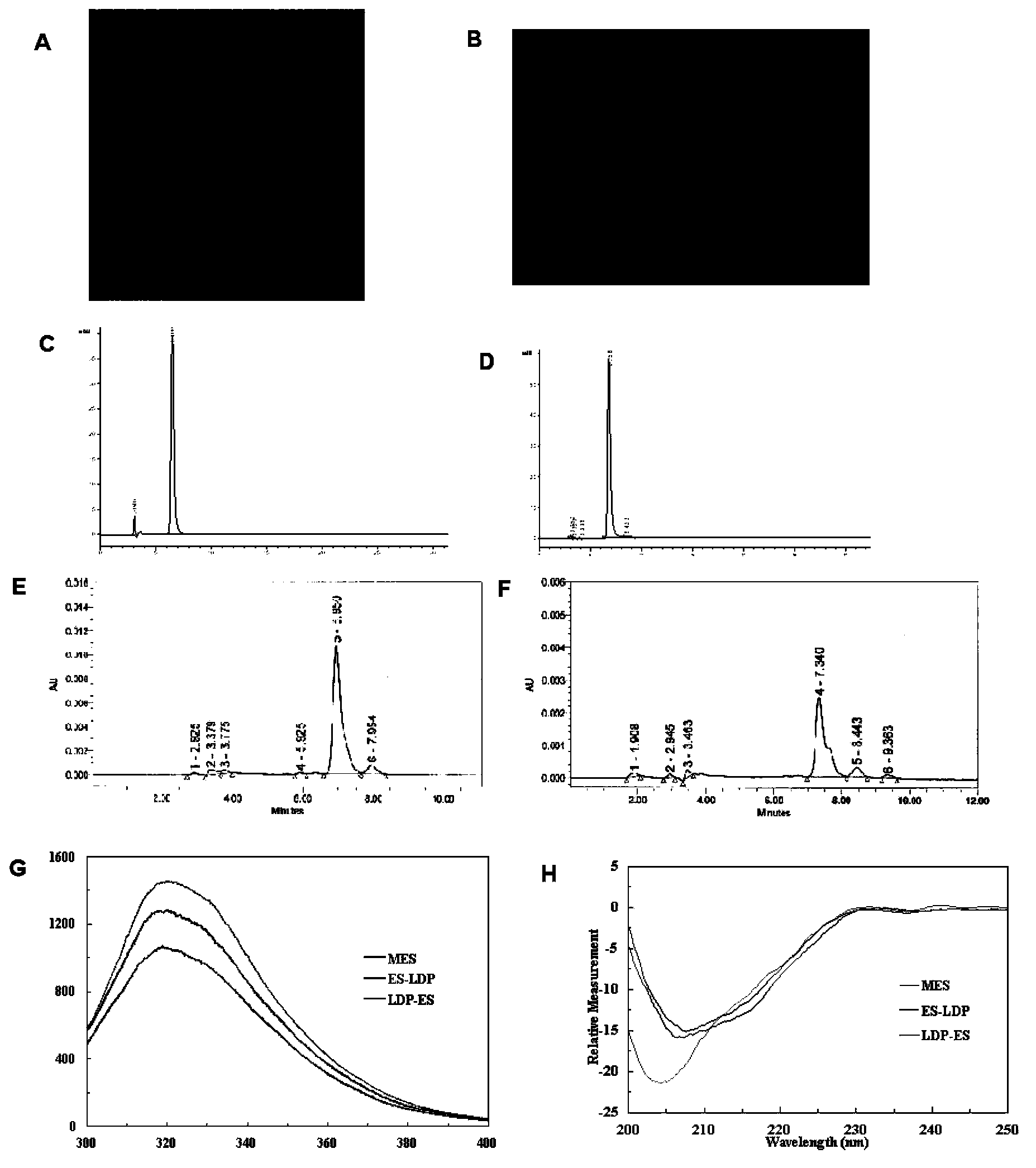
[0056] The transformed cells obtained in Example 1 were used to express the fusion protein on a large scale. by SDS-PAGE ( figure 1 B) and western blot ( figure 1 C) method to detect the expression of the fusion protein. The expressed fusion protein was purified and refolded for SDS-PAGE and HPLC analysis respectively. The result is as figure 2 Shown: figure 2 A represents the SDS-PAGE pattern of the fusion protein ES-LDP after renaturation after purification, in which: the left side is reducing electrophoresis; the right side is non-reducing electrophoresis; figure 2 B represents the SDS-PAGE profile of the fusion protein LDP-ES after renaturation after purification, in which: the left side is reducing electrophoresis; the right side is non-reducing electrophoresis; figure 2 C represents the RP-HPLC analysis of the fusion protein ES-LDP after renaturation, figure 2 D re...
Embodiment 3
[0057] Embodiment 3, the combination of fusion protein ES-LDP and LDP-ES and lidamycin chromophore
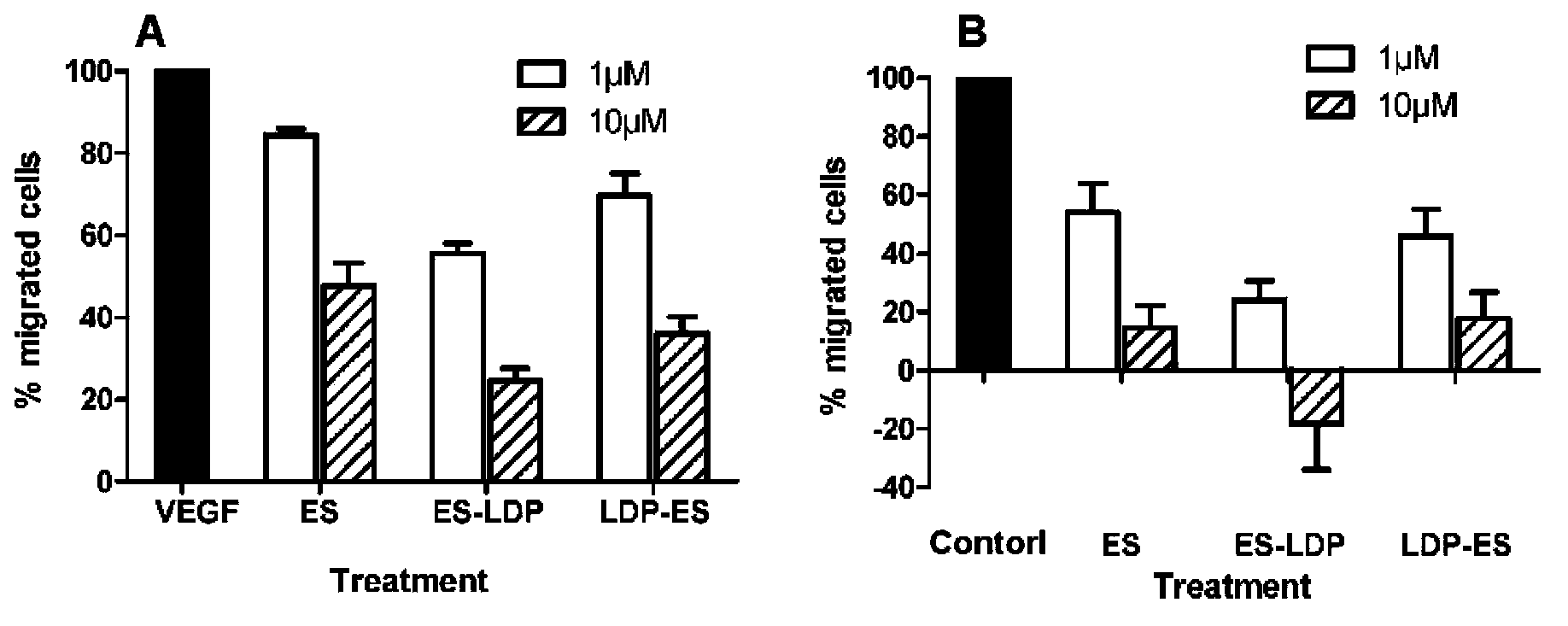
[0058] Get highly active lidamycin pure product (prepared and preserved by the Institute of Medical Biotechnology, Chinese Academy of Medical Sciences), and separate the active chromophore through a C4 column. The mobile phase is water: acetonitrile: trifluoroacetic acid (78%: 22% : 0.05%), monitor the absorbance at 350nm, and collect the lidamycin chromophore. The fusion protein obtained in Example 2 was mixed with the lidamycin chromophore at a molar ratio of 1:4, placed on a shaker and shaken slowly, and reacted at 4°C in the dark for 16 hours. The mixed solution was removed through the Sephadex G-25 column to remove the unbound lidamycin chromophore, and the absorbance value at 340nm was detected by HPLC (C4300A column) ( figure 2 E and F). The respective binding efficiencies of the two fusion proteins are calculated according to the standard curve of the lidamycin chrom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com