Recombinant herpes simplex virus capable of excreting angiostatin and endostatin protein and its application in treating lung cancer
An endostatin, angiostatin technology, applied in application, gene therapy, virus/phage and other directions, can solve the problems of short expression time, lack of specificity, host infection, etc., achieve good genetic stability, inhibit virus growth, Infectious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: Construction of recombinant virus AE618
[0063] (a) the preparation method of the F strain viral DNA of HSV-1 virus:
[0064] The F strain of HSV-1 virus used for recombination was purchased from ATCC Company of the United States. The virus was used to infect cultured Vero cells (ATCC, USA) under the condition of MOI=1. After 24-36 hours, when most of the cells showed cytopathic changes (CPE), the cells were collected and transferred to a 15ml centrifuge tube to remove the cell culture medium by centrifugation. Add 0.3ml of cell lysate (containing: 100Mm NaCl, 10Mm Tris.HCl pH 8, 25Mm EDTA pH8 and 0.5% SDS) to lyse the infected cells and treat them with RNaseA (10mg / ml) at 65°C for 1 hour. The phenol was then extracted with an equal volume of phenol / chloroform (1:1) mixture, the aqueous phase solution containing DNA was transferred to a new centrifuge tube, and 1 / 2 volume of 7.5M ammonium acetate and 2 times the volume of absolute alcohol were added. ...
Embodiment 2
[0088] Example 2: Detection of fusion protein expression in cancer cells infected with AE618
[0089] Before infection, the lung cancer A549 cells (1×10 6 )4 hours. Then they were infected with G207, AE618, or blank control (each infection titer M.O.I.=1.0), and the infected cells were cultured for 24 hours. Rinse the cultured cell samples twice with PBS, then gently scrape the cells from the plate, and use cell lysis buffer (50mM Tris-HCl pH 7.5, 150mM NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS) to lyse the cells. Whole cell lysates were boiled and protein concentrations were determined by the Bradford method (Bio-Rad, Hercules, CA). Take an equal amount of protein (100 μg) samples and perform electrophoresis separation on a 15% acrylamide gel under reducing conditions. The protein after electrophoresis was transferred to Immobilon-P transfer membrane (Millipore, Bedford, MA), and then the membrane was placed in 5% skimmed milk powder to block, and at 4°C, it was...
Embodiment 3
[0091] Embodiment 3: Detection of the cell lysis effect of recombinant virus AE618
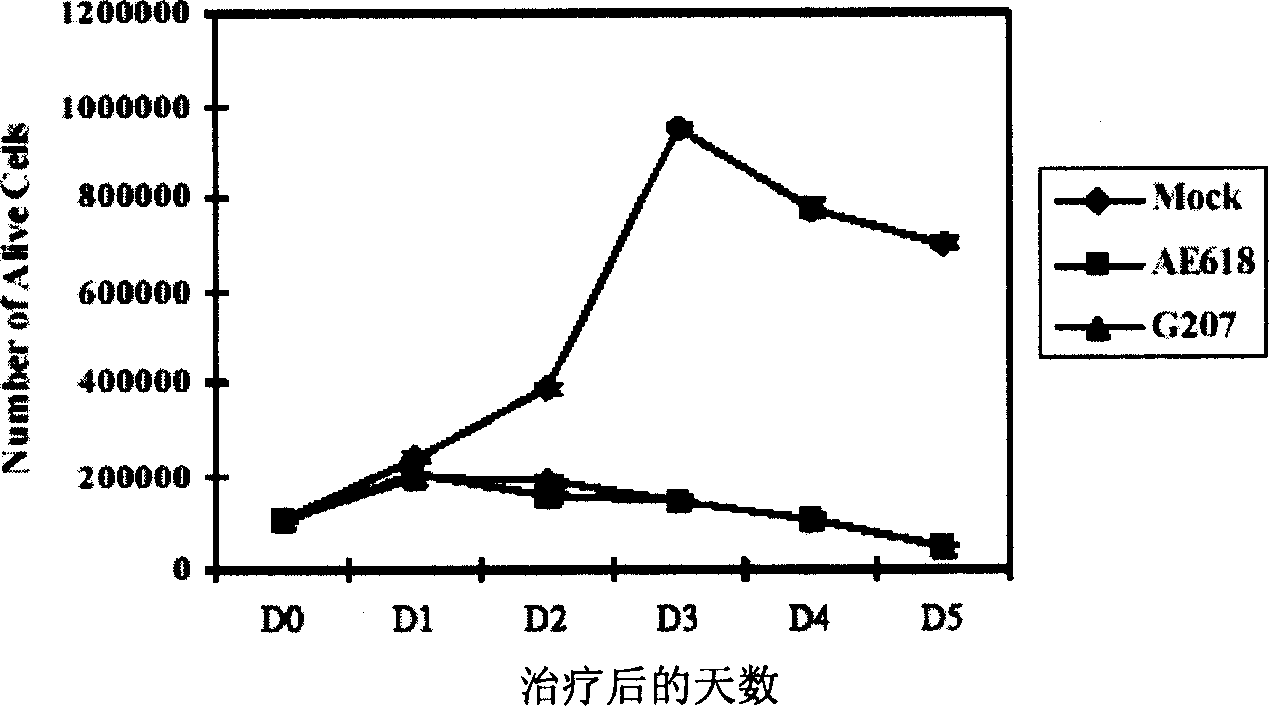
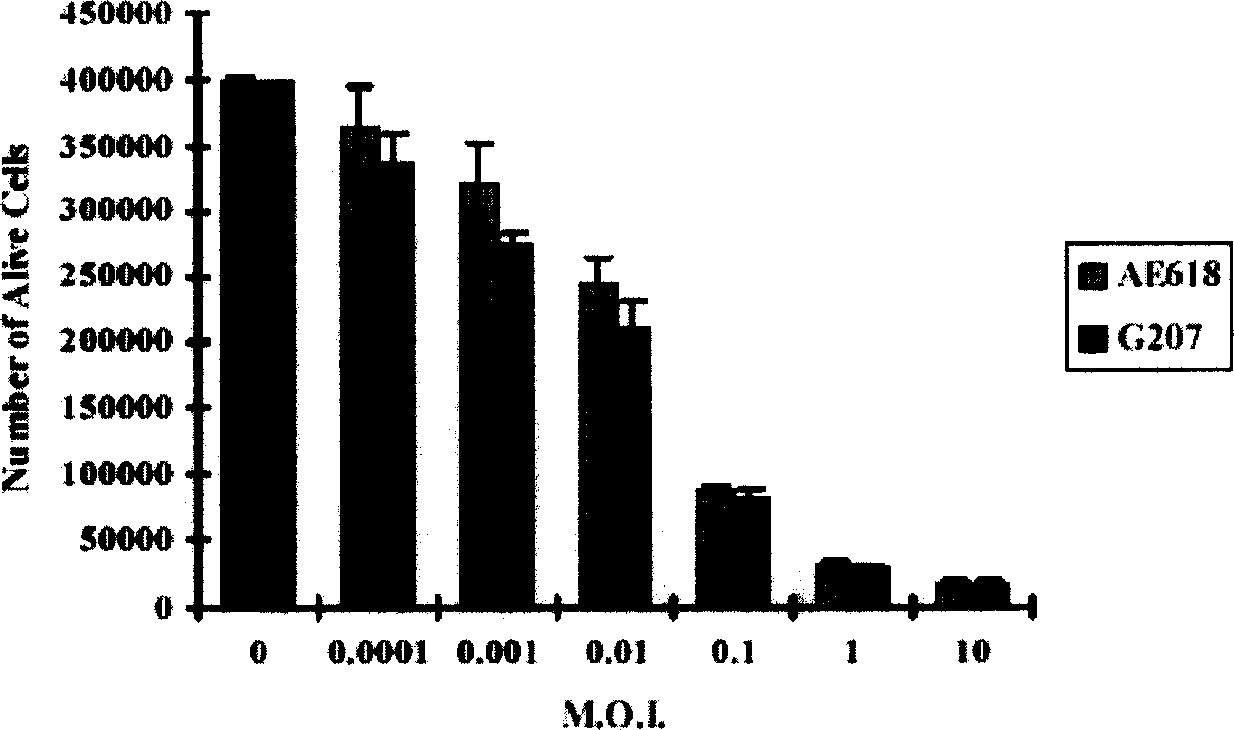
[0092] The A549 and H460 lung cancer cell lines (1×10 5 ) were placed in a 12-well tissue culture plate, cultured at 37°C for 4 hours, and then the cells were infected with various concentrations of G207 and AE618 recombinant viruses and a blank control, and the cells were continued to be cultured for several days. After trypsinization, cells were collected and resuspended in PBS solution. Add an equal volume of PBS containing 0.1% trypan blue to the cell suspension. Viable cell counts were then performed using a hemocytometer. All cell counts are the average of 3 sample counts.
[0093] The cell count results of H460 lung cancer cells from 1 to 5 days after infection showed that compared with the blank control, the number of viable cells in the group treated with virus from the second day after infection decreased significantly (p0.05, FIG. 2A ).
[0094] In addition, the H460 cell line...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com