Anti-angiogenic peptides from the N-terminus of endostatin
An anti-angiogenesis and angiogenesis technology, applied in the direction of specific peptides, connective tissue peptides, peptide/protein components, etc., can solve problems such as the unknown mechanism of endostatin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
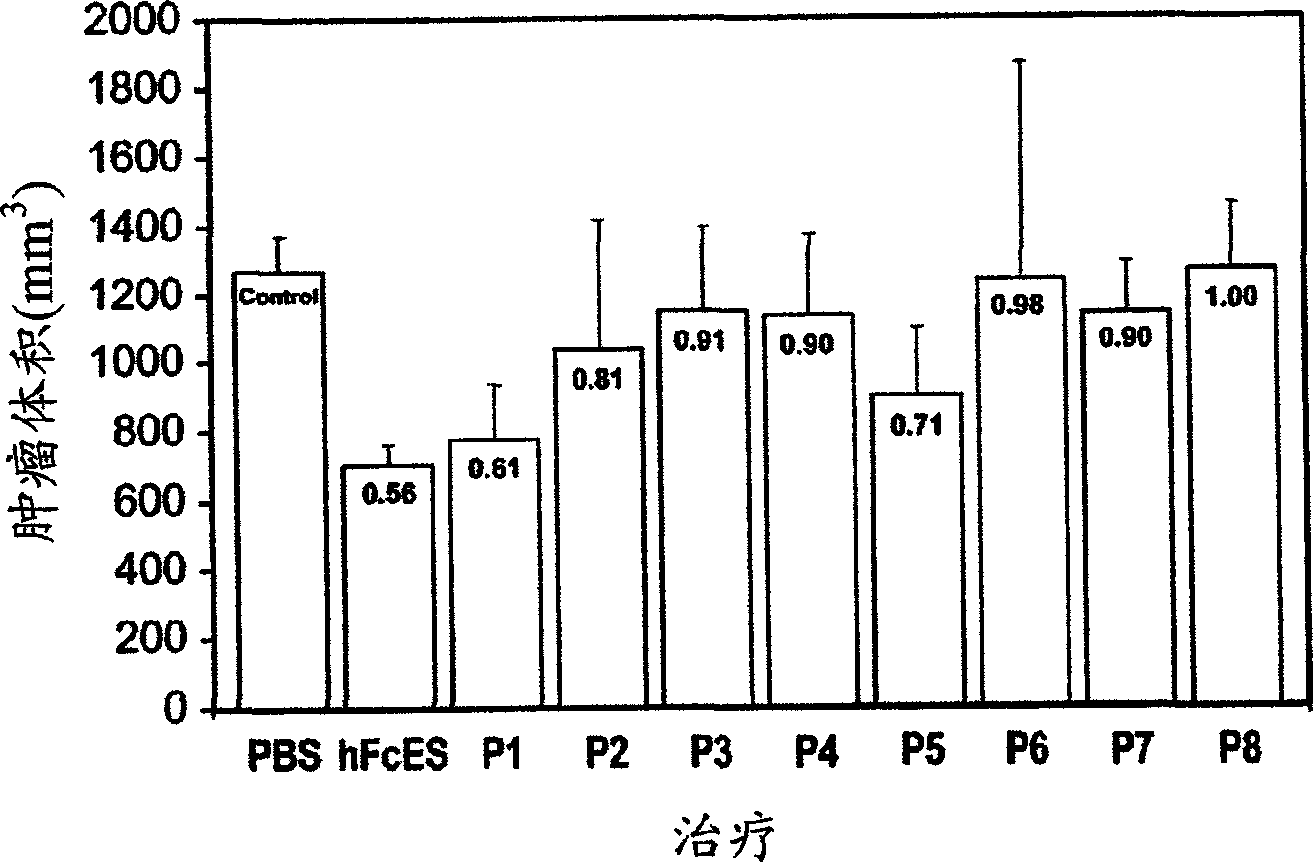
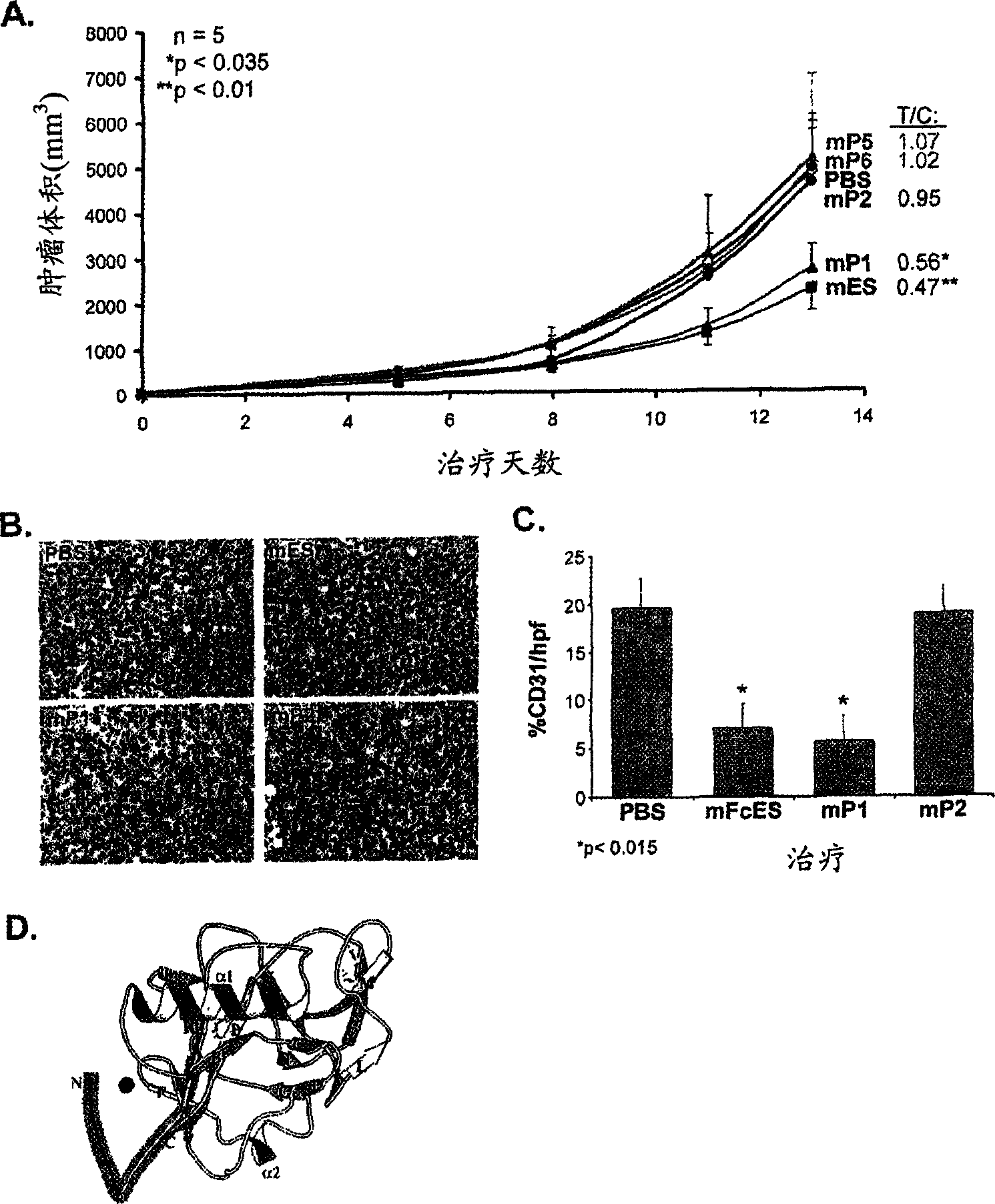
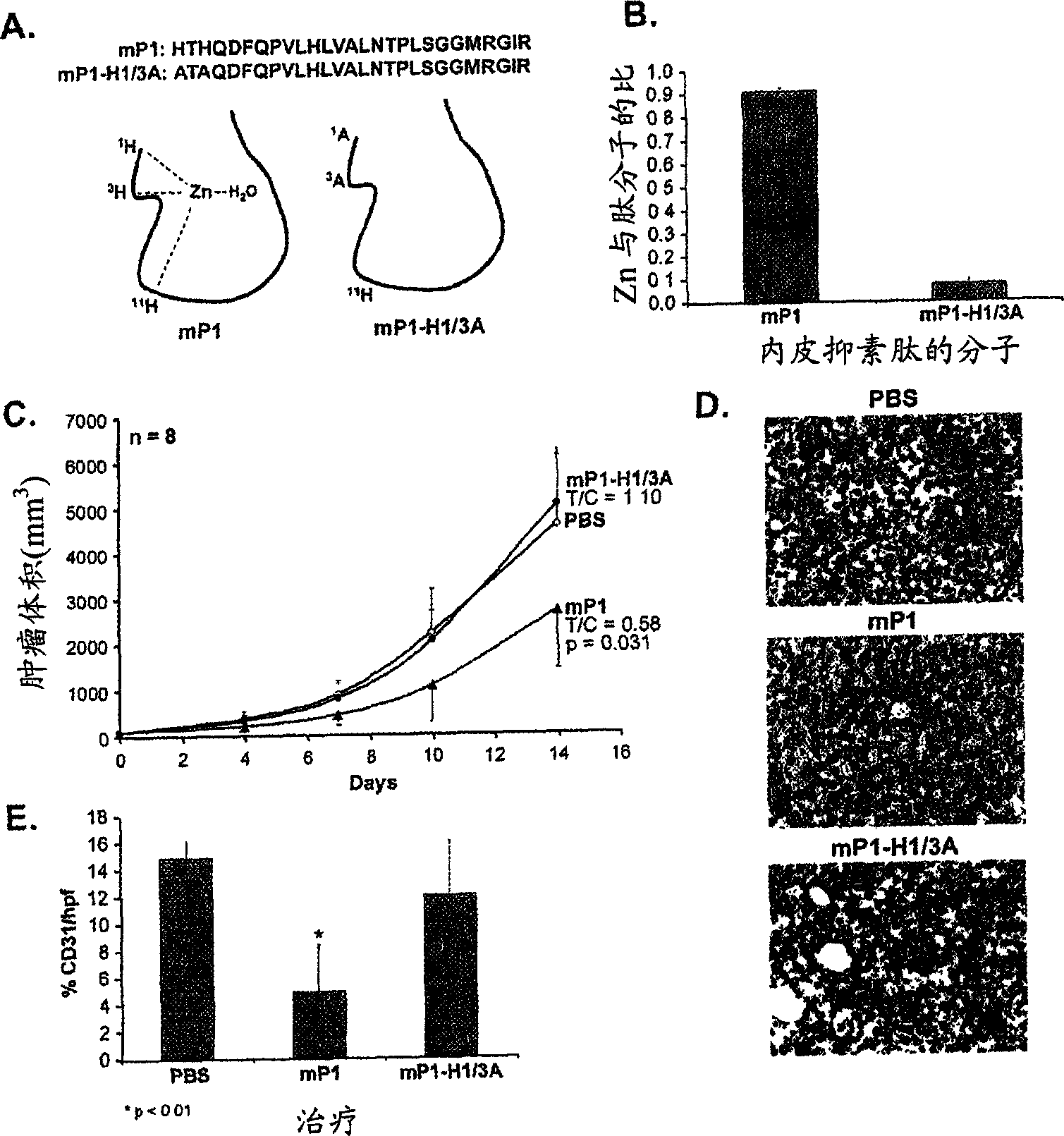
[0288] Example 1: Identification of a 27 amino acid endostatin peptide that produces antitumor activity
[0289] Overlapping peptides of 24-27 amino acids derived from both mouse endostatin and human endostatin were synthesized (Table 1).
[0290] Table 1. Overlapping Mouse and Human Endostatin Peptides
[0291] name sequence
[0292] mouse peptide
[0293] mP1: HTHQDFQPVLHLVALNTPLSGGMRGIR (SEQ ID NO: 4);
[0294] mP2: MRGIRGADFQAFQQARAVGLSGTFR (SEQ ID NO: 136);
[0295] mP3: TFRAFLSSRLQDLYSIVRRADRGSV (SEQ ID NO: 137);
[0296] mP4: GSVPIVNLKDEVLSPSWDSLFSGSQ (SEQ ID NO: 138);
[0297]mP5: GSQGQVQPGARIFSFDGRDVLRHPA (SEQ ID NO: 139);
[0298] mP6: HPAWPQKSVWHGSDPSGRRLMESY (SEQ ID NO: 140);
[0299] mP7: ETWRTETTGATGQASSLLSGRLLEQ (SEQ ID NO: 141);
[0300] mP8: KAASAHNSYIVLAIENSFMTSFSKKK (SEQ ID NO: 142).
[0301] Human peptide
[0302] hP1 1 HSHRDFQPVLHLVALNSPLSGGMRG 25 (SEQ ID NO: 6)
[0303] hP2 23 MRGIRGADFQ A FQQARAVGLAGTFR 47 (SEQ ID NO: 143)
[0304] hP345TFRA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com